Lunan Xinkang® and other 9 preparations were approved for marketing by the Macao Health Bureau
New Media Department Yu Jie, Duan Yuqing March 10, 2023
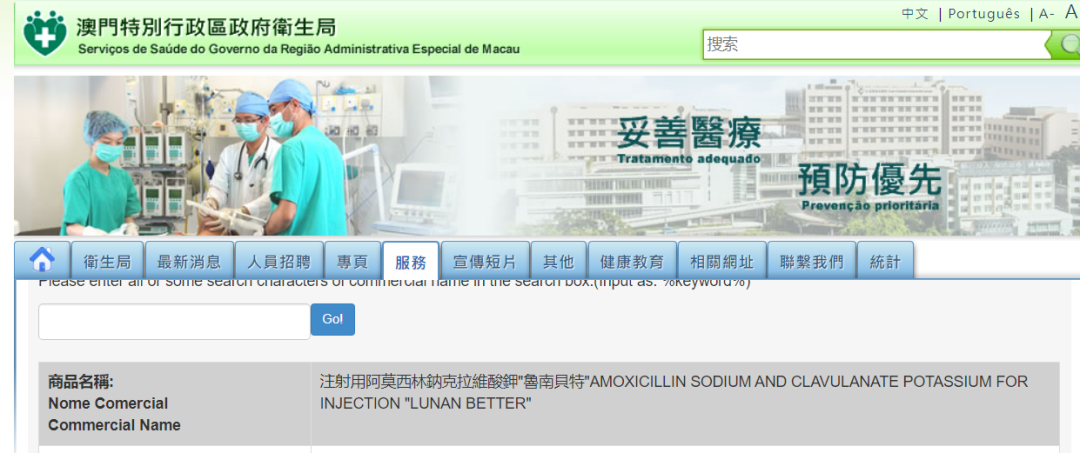
Recently, nine "historic preparations" including isosorbide mononitrate tablets, amoxicillin clavulanate potassium for injection, amoxicillin clavulanate potassium tablets, montelukast sodium chewable tablets, riluzole tablets, rosuvastatin calcium tablets, sevoflurane inhalation, and isoflurane inhalation from Lunan Better Pharmaceutical Co., Ltd, a subsidiary company of Lunan Pharma, have been approved by the Health Bureau of the Macao Special Administrative Region Government, marking the nine preparations of Lunan Bitter Pharmaceutical Co., Ltd. enters the market of Macao Special Administrative Region.
In order to speed up the process of internationalization, Lunan Pharma has established an international drug research and development center, focusing on research and development and declaration in the global market. At present, Lunan Pharma has obtained 2 approvals for preparations in Europe, 3 approvals for preparations in the United States, 18 approvals for preparations in emerging markets, 8 CEP certificates, and submitted more than 30 DMFs in China, the United States, Europe and Japan, and more than 50 DMFs in other emerging markets ; 7 US ANDAs, 2 European MAs and 10 Chinese formulations are under review. The R&D and listing of more products will accelerate the company's pace of entering the international market and enhance the brand image and popularity of Lunan Pharma at home and abroad.
