Milrinone Injection
DescriptionFull Analysis capability
Thin Film Coating Lab
Milrinone Injection
Indication
It is suitable for acute and chronic intractable congestive heart failure caused by various reasons such as ineffective or poor effect of digitalis, diuretics and vasodilators.
Clinical pharmacology
This product is a phosphodiesterase inhibitor, which is a similar drug of amrinone, and its mechanism of action is the same as that of amrinone. Both oral and intravenous injections are effective, with both positive inotropic and vasodilating effects. However, its effect is 10 to 30 times stronger than that of ammonia. Good tolerance. The positive inotropic effect of this product is mainly to inhibit the concentration of cyclic adenosine monophosphate (cAMP) in cardiomyocytes by inhibiting phosphodiesterase, increase intracellular calcium, strengthen myocardial contractility, and increase cardiac output. It is not related to the adrenergic SS1 receptor or cardiomyocyte Na+, K+-ATPase. The vasodilatation may be directly caused by small arteries, which can reduce the anterior and posterior load of the heart, reduce left ventricular filling pressure, improve left ventricular function, increase cardiac index, but have no significant effect on mean arterial pressure and heart rate. The cardiovascular effect of milrinone is related to the dose. In small doses, it mainly manifests as positive inotropic effect. When the dose is increased and gradually reaches the maximum positive inotropic effect of steady state, the effect of dilating blood vessels can also be related to the dose. Increase and gradually strengthen. This product is safer for patients with conduction block. This product has a serious adverse reaction when taken orally, and should not be used for a long time.
Dosage
Intravenous injection: load 25-75μg/kg, slow intravenous injection for 5-10 minutes, and maintained at 0.25-1.0μg/kg per minute thereafter. The maximum daily dose does not exceed 1.13 mg/kg.
Formulation
Injection
specification
5ml: 5mg
Instruction manual
Approval date:
Date of revision: 2013-04-10 December 01, 2015 January 21, 2016
Millinon injection instructions
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: Milinong injection
(Former name: Lactic milrinone injection)
Product Name: Lunan Likang
English name: Milrinone Injection
Pinyin: Milinong Zhusheye
【Ingredients】Milinon.
Chemical name: 1,6-dihydro-2-methyl-6-oxo-[3,4'bipyridine]-5-carbonitrile.
Chemical Structure:
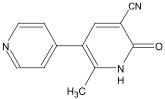
Molecular formula: C12H9N3O Molecular Weight: 211.22
Excipients: lactic acid, sodium chloride.
This product contains 10.75mg of lactic acid per bottle.
【Properties】 This product is a colorless and clear liquid.
【Indications】 It is suitable for acute and chronic refractory congestive heart failure caused by various reasons such as ineffective or poorly effective treatment of digitalis, diuretics, and vasodilators.
【Specification】 5ml: 5mg
【Dosage】
Intravenous injection: load 25-75μg/kg, slow intravenous injection for 5-10 minutes, and maintained at 0.25-1.0μg/kg per minute thereafter. The maximum daily dose does not exceed 1.13 mg/kg.
Oral: 2.5 to 7.5 mg once, 4 times a day.
【Adverse reactions】
It is rarer than ammonia. A few have headaches, ventricular arrhythmias, weakness, and decreased platelet counts. In case of overdose, there may be hypotension and tachycardia. Long-term oral administration due to large side effects can lead to increased long-term mortality and is no longer used.
【taboo】 hypotension, tachycardia, myocardial infarction with caution; renal dysfunction should be reduced.
【Precautions】
1. Heart rate, heart rate, blood pressure should be monitored during medication and the dose should be adjusted if necessary.
2, should not be used for patients with severe valvular stenosis and obstructive hypertrophic cardiomyopathy. Patients with acute ischemic heart disease should be used with caution.
3, when combined with strong diuretics, the left ventricular filling pressure can be excessively reduced, and easy to cause water, electrolyte imbalance.
4, for patients with atrial flutter, atrial fibrillation, because of increased atrioventricular conduction caused by increased ventricular rate, it is appropriate to first use digitalis preparation to control ventricular rate.
5, liver and kidney function damage with caution.
6, has not been used for myocardial infarction, pregnant women and lactating women, children, should be cautious.
【Pregnant women and lactating women】This experiment was not conducted and there is no reliable reference.
【Child medication】This experiment was not performed and there is no reliable reference.
【Geriatric Use】This experiment was not performed and there is no reliable reference.
【medicine interactions】
1, with propiamine can cause low blood pressure.
2, combined with commonly used cardiotonic, diuretic, vasodilator drugs, no adverse interactions have been seen.
3. It has an additive effect with the combination of nitrates.
4. This product has the positive inotropic effect of strengthening digitalis, so it is not necessary to stop digitalis during application.
5. Mix with furosemide and immediately produce a precipitate.
【Drug overdose】 This experiment was not performed and there is no reliable reference.
【Pharmacology and Toxicology】
This product is a phosphodiesterase inhibitor, which is a similar drug of amrinone, and its mechanism of action is the same as that of amrinone. Both oral and intravenous injections are effective, with both positive inotropic and vasodilating effects. However, its effect is 10 to 30 times stronger than that of ammonia. Good tolerance. The positive inotropic effect of this product is mainly to inhibit the concentration of cyclic adenosine monophosphate (cAMP) in cardiomyocytes by inhibiting phosphodiesterase, increase intracellular calcium, strengthen myocardial contractility, and increase cardiac output. It is not related to the adrenergic SS1 receptor or cardiomyocyte Na+, K+-ATPase. The vasodilatation may be directly caused by small arteries, which can reduce the anterior and posterior load of the heart, reduce left ventricular filling pressure, improve left ventricular function, increase cardiac index, but have no significant effect on mean arterial pressure and heart rate. The cardiovascular effect of milrinone is related to the dose. In small doses, it mainly manifests as positive inotropic effect. When the dose is increased and gradually reaches the maximum positive inotropic effect of steady state, the effect of dilating blood vessels can also be related to the dose. Increase and gradually strengthen.
This product is safer for patients with conduction block. This product has a serious adverse reaction when taken orally, and should not be used for a long time.
【Pharmacokinetics】The intravenous administration takes effect from 5 to 15 minutes, and the elimination half-life is 2 to 3 hours. The protein binding rate is 70%.
【Storage】sealed and kept in a dry place.
【Package】 Easy to fold, 2 pieces / box.
【Validity period】 24 months.
【Executive Standards】"Chinese Pharmacopoeia" 2015 Edition 2
【Approval No.】 National Pharmaceutical Standard H10970051
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 8336337 (Quality Management Department)
Fax number: 0539-8336029 (Sales) 8336338 (Quality Management Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
