Ketorolac Tromethamine Capsules
DescriptionFull Analysis capability
Thin Film Coating Lab
Ketorolac Tromethamine Capsules
Indication
This product is suitable for short-term treatment of acute and severe pain requiring opioid analgesics. It is usually used for postoperative analgesia and is not suitable for the treatment of mild or chronic pain.
Clinical pharmacology
Ketorolatrid tromethamine is a non-steroidal anti-inflammatory drug that inhibits prostaglandin biosynthesis and its biological activity is related to its S-form. Animal studies have shown that ketorolac tromethamine has an analgesic effect and no sedative or anxiolytic effects.
Dosage
oral. This product is only used for the follow-up treatment of ketorolac tromethamine injection, and the continuous administration time with intravenous or intramuscular injection is no more than 5 days
Formulation
capsule
specification
10 mg (based on ketorolac tromethamine).
Instruction manual
Approval date:
Modified date: 2010
April 30, 2014
Ketosuccinate tromethamine capsule instructions
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: ketorolac tromethamine capsules
Product Name: Nisson
English name: Ketorolac Tromethamine Capsules
Pinyin: Tongluosuan Andingsanchun Jiaonang
【Ingredients】ketorolac tromethamine.
Chemical name: (±)-5-benzoyl-2,3-dihydro-1H-pyrrolazine-1-carboxylic acid and 2-amino-2-hydroxymethyl-1,3-propanediol salt (1:1 )Complex.
Chemical Structure:
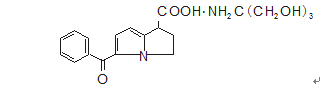
Molecular formula: C15H13NO3·C4H11NO3
Molecular weight: 376.40
【Properties】 The contents of this product are white or off-white powder.
【indications】
This product is suitable for short-term treatment of acute and severe pain requiring opioid analgesics. It is usually used for postoperative analgesia and is not suitable for the treatment of mild or chronic pain.
【Specification】 10 mg (based on ketorolac tromethamine).
【Dosage】
oral. This product is only used for the follow-up treatment of ketorolac tromethamine injection, and the continuous administration time with intravenous or intramuscular injection does not exceed 5 days. The recommended dosage for oral therapy after intravenous or intramuscular injection of this product is as follows:
Under 65 years old: intramuscular single dose 60mg, intravenous single dose 30mg or 30mg multiple administration, oral first 2 capsules, then every 4 to 6 hours orally, 1 capsule, the maximum oral dose is not more than 40mg/24 hour.
65 years of age or older, kidney injury or weight less than 50kg (110 lbs): intramuscular single dose 30mg, intravenous single dose 15mg or 15mg multiple doses, oral first 1 capsule, every 4-6 hours after Take 1 capsule orally, the maximum oral dose is no more than 40mg/24 hours.
Shortening the recommended dosing interval may result in an increased degree and frequency of adverse reactions.
【Adverse reactions】
1. Complications that may occur during clinical treatment of this product include: gastrointestinal ulcers, hemorrhage, perforation, postoperative bleeding, renal failure, allergies and allergic reactions and liver failure.
2. The clinically reported adverse reactions associated with ketorolac tromethamine are as follows:
· The incidence rate is above 1%:
Systemic: edema (4%)
Cardiovascular: Hypertension
Skin disease: itching, rash
Gastrointestinal tract: nausea (12%), dyspepsia (12%), gastrointestinal pain (13%), diarrhea (7%), constipation, bloating, abdominal pain, vomiting, stomatitis
Blood and lymphatic system: purpura
Nervous system: headache (17%), lethargy (6%), dizziness (7%), sweating
Injection site pain: In 2% of patients with multiple drug administration studies, this adverse reaction occurred.
· The incidence rate is 1% or less:
Systemic: weight gain, fever, infection, weakness
Cardiovascular: agitation, paleness, syncope, vasodilation
Skin disease: rubella
Gastrointestinal tract: gastritis, rectal bleeding, hernia, loss of appetite, increased appetite
Blood and lymphatic system: nosebleeds, anemia, eosinophilia
Nervous system: tremor, nightmare, hallucination, euphoria, extra-vertebral system symptoms, dizziness, paresthesia, depression, insomnia, nervousness, dry mouth, abnormal thinking, inability to concentrate, motor hyperactivity, paralysis
Respiratory system: difficulty breathing, pulmonary edema, rhinitis, cough
Special feeling: abnormal taste, abnormal vision, blurred vision, tinnitus, loss of hearing
Genitourinary system: hematuria, proteinuria, oliguria, urinary retention, polyuria, frequent urination
3. Improper medication or increased dose will increase the incidence of adverse reactions.
4. Monitoring of adverse reactions after listing:
Systemic: allergic reactions, laryngeal edema, edema of the tongue, angioedema, myalgia
Cardiovascular system: hypotension, flushing
Skin disease: Lyell’s syndrome, Stevens-Johnson syndrome, exfoliative dermatitis, maculopapular rash, rubella
Gastrointestinal tract: peptic ulcer, gastrointestinal bleeding, gastrointestinal perforation, black feces, acute pancreatitis, hematemesis, esophagitis.
Blood and lymphatic system: postoperative wound bleeding, reduced platelet count, reduced white blood cell count
Liver: hepatitis, liver failure, cholestasis jaundice
Nervous system: convulsions, psychosis, aseptic meningitis
Respiratory system: asthma, bronchospasm
Genitourinary system: acute renal failure, rib pain (with or without hematuria or azotemia), interstitial nephritis, hyponatremia, hyperkalemia, hemolytic uremic syndrome.
5. According to a non-randomized follow-up study of about 10,000 patients who used ketorolac tromethamine, the results showed that there was a risk of severe gastrointestinal bleeding, which was related to the dose (see table below). The phenomenon is more common in elderly patients with a daily average dose of more than 60 mg/day.
Adverse reaction monitoring table after intravenous or intramuscular injection of ketorolac tromethamine for 5 days
A. Adult patients without history of gastrointestinal perforation, ulceration, and bleeding | ||||
Patient age | Daily intravenous or intramuscular total dose | |||
≤60mg | >60 to 90mg | >90 to 120mg | >120mg | |
Under 65 years old | 0.4% | 0.4% | 0.9% | 4.6% |
65 years or older | 1.2% | 2.8% | 2.2% | 7.7% |
B. Adult patients with a history of gastrointestinal perforation, ulceration, and bleeding | ||||
Patient age | Daily intravenous or intramuscular total dose | |||
≤60mg | >60 to 90mg | >90 to 120mg | >120mg | |
Under 65 years old | 2.1% | 4.6% | 7.8% | 15.4% |
65 years or older | 4.7% | 3.7% | 2.8% | 25.0% |
【taboo】
1. Patients who are known to be allergic to this product.
2. Patients who develop asthma, urticaria or allergic reactions after taking aspirin or other non-steroidal anti-inflammatory drugs.
3. Disable the treatment of perioperative pain in coronary artery bypass surgery (CABG).
4. Patients with a history of gastrointestinal bleeding or perforation after the application of non-steroidal anti-inflammatory drugs.
5. Patients with active peptic ulcer/bleeding, or who have had recurrent ulcers/bleeds in the past.
6. Patients with severe heart failure.
【Precautions】
1. Avoid combination with other non-steroidal anti-inflammatory drugs, including selective COX-2 inhibitors.
2. According to the need to control symptoms, the use of the lowest effective dose within the shortest treatment time can minimize adverse reactions.
3. Adverse reactions to gastrointestinal bleeding, ulceration, and perforation may occur at any time during the course of treatment with all non-steroidal anti-inflammatory drugs, and the risk may be fatal. These adverse reactions may or may not be accompanied by warning symptoms, regardless of whether the patient has a history of gastrointestinal adverse reactions or a history of severe gastrointestinal events. Patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) should be careful to use non-steroidal anti-inflammatory drugs to avoid worsening the condition. When the patient takes this medicine for gastrointestinal bleeding or ulceration, the drug should be discontinued. The increased frequency of adverse reactions in elderly patients with non-steroidal anti-inflammatory drugs, especially gastrointestinal bleeding and perforation, may be fatal.
4. Clinical trials with multiple COX-2 selective or non-selective NSAIDs for up to 3 years have shown that this product may cause an increased risk of severe cardiovascular thrombotic adverse events, myocardial infarction and stroke, the risk may be fatal. All NSAIDs, including COX-2 selective or non-selective drugs, may have similar risks. Patients with cardiovascular or cardiovascular risk factors are at greater risk. Even if there are no previous cardiovascular symptoms, doctors and patients should be alert to the occurrence of such events. Patients should be informed of the signs and/or signs of severe cardiovascular safety and the steps that should be taken if they occur.
Patients should be alert to symptoms and signs such as chest pain, shortness of breath, weakness, speech ambiguity, and should seek medical help immediately after any of the above symptoms or signs.
5. Like all non-steroidal anti-inflammatory drugs (NSAIDs), this product can cause new high blood pressure or exacerbate existing hypertension symptoms, any of which can lead to an increase in the incidence of cardiovascular events. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients taking thiazides or myeloid diuretics may affect the efficacy of these drugs. Non-steroidal anti-inflammatory drugs (NSAIDs), including this product, should be used with caution in patients with hypertension. Blood pressure should be closely monitored during the start of treatment and throughout the treatment.
6. Patients with a history of hypertension and / or heart failure (such as fluid retention and edema) should be used with caution.
7. NSAIDs, including this product, may cause fatal, severe skin reactions such as exfoliative dermatitis, Stevens Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). These serious events can occur without warning. Patients should be informed of the signs and symptoms of severe skin reactions. This product should be discontinued the first time a skin rash or other signs of an allergic reaction occur.
【Pregnant women and lactating women】This product can be banned from pregnant women through the placental barrier. This product can be secreted into the milk, although the amount is very small, but lactating women still have to be banned.
【Child medication】This product is not recommended for children under 2 years of age. This product is only used in children by single intravenous or intramuscular injection. The intramuscular dose is not more than 30mg, and the intravenous dose is not more than 15mg.
【Geriatric Use】 The elderly should be used with special caution or reduced dosage.
【medicine interactions】
1. There is no research data to confirm the interaction between this product and warfarin, digoxin, salicylate and heparin. However, patients who use anticoagulants should be extremely careful when giving this product. The patient was closely observed.
2. The oral preparation of this product and the combination of Bingshushu can reduce the clearance of ketorolac and significantly increase the plasma concentration of ketorolac (the total AUC increased from 5.4μg/h/ml to 17.8μg/h/ml). , about three times higher), the half-life is extended by about 2 times, from 6.6 hours to 15.1 hours. Therefore, this product is forbidden to be combined with Bingshu.
3. For the healthy subjects with normal blood volume, the diuretic effect of furosemide is reduced by about 20%.
4. It has been reported that the simultaneous administration of methotrexate and some non-steroidal anti-inflammatory drugs will reduce the clearance rate of methotrexate and increase its toxicity. However, the effect of this product on the clearance rate of methotrexate has not been studied.
5. It has been reported that prostaglandin synthesis inhibitors inhibit the clearance of lithium in the kidney, resulting in an increase in plasma lithium concentration. At present, there is no research data on the effect of this product on plasma lithium, but the occurrence of elevated plasma lithium concentration in the use of ketorolac tromethamine has been reported.
6. This product may interact with non-depolarizing muscle relaxants, resulting in apnea, but there is still no official research data in this regard.
7. The combination of this product and ACE inhibitor has the possibility of increasing renal damage, especially for patients with blood loss.
8. Epilepsy may occur when this product is combined with antiepileptic drugs (phenytoin, carbamazepine), but this possibility is minimal.
9. This product has the possibility of causing hallucinations when the drug is combined with neurological drugs (fluoxetine, tivatol, and alprazolam).
10. There is no adverse interaction between this product and morphine in the treatment of postoperative pain; however, do not mix this product with morphine in the same syringe.
11, no animal or human test to prove whether this product induces or inhibits liver enzymes, liver enzymes affect the metabolism of ketorolac tromethamine or other drugs.
【Drug overdose】
This product, like other non-steroidal anti-inflammatory drugs, may cause general symptoms such as lethargy, lethargy, nausea, vomiting, and upper abdominal pain when used in excess. It is usually eliminated by supportive care. Gastrointestinal bleeding may occur with excessive use of this product. Hypertension, acute renal failure, respiratory depression, coma, and allergic reactions occur in rare cases.
It has been reported that excessive injection of this product caused abdominal pain and peptic ulcer, and returned to normal after stopping the drug. Metabolic acidosis has also been reported. A single overdose of ketorolac tromethamine showed abdominal pain, nausea, vomiting, asthma, peptic ulcer and/or erosive gastritis, renal dysfunction, and these symptoms disappeared after stopping the drug.
Patients who use this product in excess must be given symptomatic supportive care. No detoxification is specified. Hemodialysis does not remove this product well.
【Pharmacology and Toxicology】
Pharmacological action
Ketorolatrid tromethamine is a non-steroidal anti-inflammatory drug that inhibits prostaglandin biosynthesis and its biological activity is related to its S-form. Animal studies have shown that ketorolac tromethamine has an analgesic effect and no sedative or anxiolytic effects.
Toxicological research
Genotoxicity
Ketone tromethamine Ames test, extra-program DNA synthesis and repair test, positive mutation test, mouse micronucleus test results were negative, Chinese hamster ovary cell chromosome aberration test results were positive.
Reproductive toxicity
Male and female rats were orally administered with ketorolac tromethamine 9 mg/kg (0.9 times for human AUC) and 16 mg/kg (1.6 times for human AUC), respectively, for no damage to fertility. Rabbits and rats were orally administered ketorolac tromethamine 3.6 mg/kg (0.37 times human AUC) and 10 mg/kg (1.0 times human AUC) during the teratogenic sensitive period, and no teratogenic effects were observed. Oral administration of ketorolac tromethamine 1.5 mg/kg (0.14 times human AUC) in the perinatal period caused dystocia, and the mortality rate of pups was higher.
Carcinogenicity
Mice were orally administered with ketorolac tromethamine 2 mg/kg/day for 18 consecutive months, and rats were orally administered 5 mg/kg/day for 24 consecutive months without carcinogenicity.
【Pharmacokinetics】
The main component of this product, ketorolac tromethamine, is a racemate composed of levorotatory and dextrorotatory isomers, and its right-handed S(+) isomer has an analgesic effect.
A comparative study of the pharmacokinetic properties of ketorolac tromethamine showed that for adults, the bioavailability of oral and intramuscular injection is equivalent to intravenous injection. The in vivo, intramuscular, and intravenous in vivo clearance rates were unchanged for adults who received a single dose of the recommended dose at different routes of administration. This indicates that single and multiple oral, intramuscular, and intravenous administrations of adults have a linear pharmacokinetic profile. Increasing the dose, the blood concentration in the body increases linearly.
Absorption: The oral absorption rate of this product can reach 100%. However, high-fat food can affect the oral absorption of this product, which lowers the peak concentration of plasma and delays the peak time by about 1 hour; the antacid does not affect the absorption of this product.
Distribution: This product has a higher binding rate to serum proteins after absorption, up to 99% at therapeutic concentrations. After single dose administration of this product, the maximum volume of distribution (Vβ) is 13 liters.
Metabolism: This product is mainly metabolized by the liver.
Excretion: This product is mainly excreted by the kidney; about 92% of the drug is discharged through the kidney with urine, 40% of which is metabolite, and 60% is ketorolac. There is also approximately 6% of the drug administered excreted from the feces. Studies have shown that the total clearance rate of 30 mg ketorolac tromethamine in normal adults (n=37) is 0.030 (0.017-0.051) L/h/kg. The ketorolac tromethamine L-isomer clearance was 2 times faster than the dextro isomer, and the clearance was independent of the route of administration. This means that the plasma concentration ratio of the left and right isomers will decrease over time. Between the isomers in the body, the configuration of the isomers can hardly be converted to each other.
The half-life of the ketorolac tromethamine L-isomer is about 2.5 hours (SD ± 0.4), the half-life of the dextro isomer is about 5 hours (SD ± 1.7), and the half-life of the racemate is 5 to 6 hours. Within the scope.
Accumulation: This product is tested on healthy people (n=13). This product is administered intravenously every 6 hours according to the clinical recommended dose for 5 consecutive days. It can reach steady blood after the 4th administration. There was no significant difference in drug concentration, Cmax between day 1 and day 5.
There are no reports of drug accumulation in special populations (aged patients, children, renal failure or liver disease patients).
Pharmacokinetics of special populations
Elderly patients: According to the single-dose study data, the half-life of the ketorolac tromethamine racemate in the elderly (65-78 years old) is 5-7 hours longer than that of young healthy volunteers (24-35 years old). There was almost no difference in Cmax between the two groups.
Children: 10 children aged 4-8 years were given a single dose of ketorolac tromethamine (0.5 mg/kg) with an average half-life of 6 hours (3.5-10 hours). The distribution volume and clearance rate of ketorolac in pediatric patients is twice that of adults. There are no pharmacokinetic studies of intramuscular injections of ketorolac tromethamine in pediatric patients.
Patients with renal insufficiency: According to the single-dose study data, the half-life of ketorolac tromethamine in patients with renal injury is 6 to 19 hours, and the degree of renal injury determines the half-life of the product.
Hepatic insufficiency: The half-life, AUC and Cmax values of ketorolac tromethamine in 7 patients with liver disease were not significantly different from those in healthy volunteers.
Clinical drug research
Adult patients: double-blind, single-repeated, parallel design for patients with moderate to severe pain in general surgery (ortrology, obstetrics and laparotomy) and oral surgery (smart extraction) To study the postoperative analgesic effect of intramuscular injection, intravenous injection and oral ketorolac tromethamine. The control group received meperidine or morphine intramuscularly, or intravenously via a PCA pump. The results are as follows:
For patients receiving short-term treatment, intramuscular injection of ketorolac tromethamine or morphine for 3 days (a few patients for 5 days), ketoacid tromethamine 30 mg analgesic effect between morphine 6mg and 12mg, in the muscle The first hour after the injection of ketorolac tromethamine began to produce an analgesic effect. When injecting ketorolac tromethamine as needed, the 30 mg analgesic effect is equivalent to one or two intravenous injections of morphine 4 mg. And ketorolac tromethamine has a longer duration of action than the comparator meperidine or morphine.
Few clinically controlled trials have been given ketorolac tromethamine for 5 consecutive days, as most patients only administer 3 days or less. The adverse effects of intravenous injection of ketorolac tromethamine were similar to those of intramuscular injection because the pharmacokinetics and bioequivalence (AUC, clearance, plasma half-life) of the two routes of administration were similar.
Pediatric patients: Single-dose intramuscular or intravenous ketorolac tromethamine in pediatric patients can reduce the amount of opioid analgesics compared with the placebo group. This confirms that ketorolac has an analgesic effect on pediatric patients.
Clinical study of combined opioids
Clinical studies have shown that ketorolac tromethamine combined with opioids can greatly reduce the amount of opioids used in the treatment of postoperative pain, and the combined analgesic effect is more obvious. This combination is effective for patients who may have opioid complications. However, ketorolac tromethamine should not be mixed with opioids in the same syringe.
【Storage】 shading, sealing, and storing in a dry place.
【Packing】 Aluminum-plastic packaging, packaging specifications are 24 / box, 36 / box, 48 / box.
【Validity Period】 Tentatively for 24 months.
【Executive Standards】National Food and Drug Administration National Drug Standard YBH40742005-2014Z
【Approval No.】 National Drug Standard H20052633
【manufacturer】
Company Name: Shandong New Times Pharmaceutical Co., Ltd.
Production address: No. 1 Outer Ring Road, Feixian County, Shandong Province
Postal code: 273400
Phone number: 0539-8336336 (Sales) 5030608 (Quality Management Department)
Fax number: 0539-5030900
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
