Omeprazole Enteric-coated Capsules
DescriptionFull Analysis capability
Thin Film Coating Lab
Omeprazole Enteric Capsules
Suitable for gastric ulcer, duodenal ulcer, stress ulcer, reflux esophagitis and Zhuo-Eye syndrome (gastrin).
Product Details
Indication
Suitable for gastric ulcer, duodenal ulcer, stress ulcer, reflux esophagitis and Zhuo-Eye syndrome (gastrin).
Clinical pharmacology
Proton pump inhibitors. This product is a fat-soluble weakly basic drug, which is easily concentrated in an acidic environment. Therefore, it can be specifically distributed in the secretory tubules of gastric mucosal cells after oral administration, and converted into an active form of sulfenamide in this high acid environment. And then through the disulfide bond and the irreversible binding of the thiol group of the H+, K+-ATPase (also known as proton pump) in the secretory membrane of the parietal cell to form a complex of the sulfinamide and the proton pump, thereby inhibiting the activity of the enzyme, The last step of blocking gastric acid secretion, so this product has a strong and lasting inhibitory effect on gastric acid secretion caused by various reasons.
Dosage
Oral, not chewable. (1) Peptic ulcer: 20mg once (1 capsule at a time), 1 or 2 times a day. Swallow every morning or morning and evening, the course of gastric ulcer is usually 4 to 8 weeks, and the course of duodenal ulcer is usually 2 to 4 weeks... (see instructions)
Formulation
capsule
specification
20mg
Instruction manual
Approval date:
Date of revision: 2015-11-25 December 01, 2015
Omeprazole enteric capsule instructions
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: Omeprazole enteric capsule
English name: Omeprazole Enteric Capsules
Chinese Pinyin: Aomeilazuo Changrong Jiaonang
【ingredients】omeprazole
Chemical name: 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)-methyl]-sulfinyl]-1H-benzimidazole
Chemical Structure:
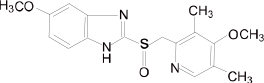
Molecular formula: C17H19N3O3S Molecular weight: 345.42
【Properties】
The contents of this product are white or off-white enteric pellets or granules.
【indications】
Suitable for gastric ulcer, duodenal ulcer, stress ulcer, reflux esophagitis and Zhuo-Eye syndrome (gastrin).
【Specification】20mg
【Usage and Dosage】 Oral, not chewable.
(1) Peptic ulcer: 20mg once (1 capsule at a time), 1 or 2 times a day. Swallow every morning or morning and evening, the course of gastric ulcer is usually 4 to 8 weeks, and the course of duodenal ulcer is usually 2 to 4 weeks.
(2) Reflux esophagitis: once 20 ~ 60 mg (1 to 3 capsules at a time), 1 or 2 times a day. Swallow in the morning or morning and evening, usually for 4 to 8 weeks.
(3) Zhuo-Ai syndrome: 60mg once (3 capsules at a time), once a day, the total daily dose can be adjusted to 20 ~ 120mg (1 ~ 6 capsules) according to the condition, if the total dose per day needs to exceed 80mg (4 capsules) should be divided into two doses.
【Adverse reactions】
This product is well tolerated. Common adverse reactions are diarrhea, headache, nausea, abdominal pain, flatulence and constipation, occasionally elevated serum aminotransferase (ALT, AST), rash, dizziness, lethargy, insomnia, etc., the above adverse reactions are usually light, can automatically disappear . For long-term treatment, gastric mucosal cell proliferation and atrophic gastritis can occur in some cases.
【Contraindications】Those who are allergic to this product and those with severe renal insufficiency are prohibited.
【Precautions】
1. When treating gastric ulcer, the possibility of ulcerative gastric cancer should be ruled out first, because treatment with this product can alleviate its symptoms and delay treatment.
2, liver and kidney dysfunction are used with caution.
3. This product is an enteric-coated capsule. Please take care not to chew it when taking it to prevent the drug particles from being released in the stomach too early and affecting the curative effect.
【Pregnant women and lactating women】Although animal experiments have shown that this product has no fetal toxicity or teratogenic effects, it is generally not used for pregnant women, and should also be used with caution in lactating women.
【Children's medication】There is no experience in children's medication, and children are not recommended.
【Geriatric Use】This experiment was not performed and there is no reliable reference.
【medicine interactions】
This product can delay the elimination of drugs metabolized by the liver, such as diazepam, phenytoin, warfarin, nifedipine, etc. When this product is used together with the above drugs, the latter should be reduced.
【Drug overdose】This experiment was not performed and there is no reliable reference.
【Pharmacology and Toxicology】
Proton pump inhibitors. This product is a fat-soluble weakly basic drug, which is easily concentrated in an acidic environment. Therefore, it can be specifically distributed in the secretory tubules of gastric mucosal cells after oral administration, and converted into an active form of sulfenamide in this high acid environment. And then through the disulfide bond and the irreversible binding of the thiol group of the H+, K+-ATPase (also known as proton pump) in the secretory membrane of the parietal cell to form a complex of the sulfinamide and the proton pump, thereby inhibiting the activity of the enzyme, The last step of blocking gastric acid secretion, so this product has a strong and lasting inhibitory effect on gastric acid secretion caused by various reasons.
【Pharmacokinetics】
After oral administration, it is absorbed by the small intestine and takes effect within 1 hour. The blood concentration reaches a peak at 0.5-3.5 hours, and the effect lasts for more than 24 hours. It can be distributed to liver, kidney, stomach, duodenum, thyroid and other tissues. Easy to pass through the placenta. Usually, the single-dose bioavailability is about 35%, the multi-dose can be increased to about 60%, the plasma protein binding rate is 95% to 96%, the plasma half-life is 0.5 to 1 hour, and the chronic liver disease is 3 hours. This product is metabolized in the body by the liver microsomal cytochrome P450 oxidase system, 80% of metabolites are excreted in the urine, and the rest is excreted from the feces after bile secretion.
【Storage】Shade, seal and store in a dry place.
【Packing】double aluminum packaging; 7 / box, 14 / box, 28 / box.
【Validity Period】 24 months
【Executive Standards】 "Chinese Pharmacopoeia" 2015 Edition 2
【Approval No.】 National Drug Standard H20033560
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 8336337 (Quality Management Department)
Fax number: 0539-8336029 (Sales) 8336338 (Quality Management Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
