
Latanoprost Eye Drops
DescriptionFull Analysis capability
Thin Film Coating Lab
Latanoprost Eye Drops
Indication
It is suitable for patients with open-angle glaucoma and ocular hypertension to reduce intraocular pressure, and can not tolerate other adverse intraocular pressure drugs or poor efficacy (multiple measurements can not meet the target intraocular pressure reduction standard).
Clinical pharmacology
Latanoprost is a novel phenyl-substituted propyl ester prostaglandin F2a that is a selective F2a receptor agonist. This product is the premise drug in the form of isopropyl ester. It is absorbed through the cornea and hydrolyzed into a biologically active free acid by the action of esterase to increase the scleral pigment efflux of aqueous humor to reduce intraocular pressure. This product can also make glaucoma patients usually block the eyeball trabecular structure screen.
Dosage
Eye drops, once a day, one drop at a time, it is best to drop in the eyes at night.
Formulation
Eye drops
specification
2.5 ml: 0.125 mg.
Instruction manual
Approval date: May 16, 2007
Modified date:

Latanoprost eye drops instructions
Please read the instructions carefully and use the approval date under the guidance of a physician.
【Drug Name】
Common name: latanoprost eye drops
English name: Latanoprost Eye Drops
Pinyin: Latanqianliesu Diyanye
【ingredients】 latanoprost.
Chemical name: Z-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5- Isobutyric acid isopropyl ester.
Chemical Structure:
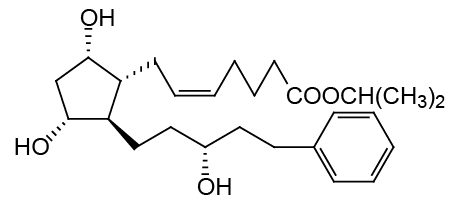
Molecular formula: C26H40O5
Molecular weight: 432.58
【Properties】 This product is a colorless or almost colorless clear liquid.
【indications】
It is suitable for patients with open-angle glaucoma and ocular hypertension to reduce intraocular pressure, and can not tolerate other adverse intraocular pressure drugs or poor efficacy (multiple measurements can not meet the target intraocular pressure reduction standard).
【Specification】2.5ml: 0.125mg.
【Usage and Dosage】 Eye drops, once a day, one drop at a time, it is best to drop in the eyes at night.
【Adverse reactions】
This product is usually well tolerated, occasionally blurred vision, burning pain, stinging, conjunctival hyperemia, transient punctate corneal erosion and foreign body sensation. Some patients also experience changes in eyelashes (length, density, color, direction), darkening of the eyelids, and brown pigmentation of the iris (7% after 6 months and 16% after 12 months). Increased pigmentation is more common in people with green-brown, blue-brown-brown, or yellow-brown irises, and is rare in people of pure blue, blue-gray, or green iris. This is caused by the stimulation of melanin formation, and the progression can be stopped after stopping the drug, but the pigmentation cannot be reversed.
【taboo】
It is banned from highly sensitive latanoprost, benzalkonium chloride and other ingredients in the formulation.
Patients who are pregnant, lactating, severe asthma or during periods of inflammatory and hyperemic eyes are banned.
【Precautions】
1 This product can cause pigmentation changes, the most frequently reported changes are to increase the pigmentation of the iris and eyelids, increase the pigmentation and growth of the eyelashes, these changes are permanent.
2 During pregnancy, lactation, severe asthma or inflammatory eyes during the eyes, patients are prohibited, children are not recommended.
3 This product is not suitable for the treatment of angle-closure or congenital glaucoma, pigmented glaucoma and open angle glaucoma of pseudomorphosis.
4 This product has synergistic effect with other anti-glaucoma drugs. Other eye drops should be used at least 5 minutes apart.
5 Wearing a contact lens should first remove the lens, drip the drug for 15 minutes before wearing the lens.
6 If you forget to take the medicine, you should use it immediately when you think about it. If you still use it regularly, don't order double doses of medicine next time.
【Pregnant women and lactating women】gestation, lactation women are prohibited.
【Child medication】 This experiment was not performed and there is no reliable reference.
【Geriatric Use】 Safety and effectiveness are not different from adults and can be used in clinical doses.
【medicine interactions】
This product has synergistic effect with other anti-glaucoma drugs. Other eye drops should be used at least 5 minutes apart.
【Drug overdose】
In addition to overdose, eye irritation, conjunctival and surrounding tissue edema, the rest is not clear. If the drug is found to be overdose, coping with the disease.
【Pharmacology and Toxicology】
Mechanism of action: Latanoprost is a novel phenyl-substituted propyl ester prostaglandin F2a, a selective F2a receptor agonist. This product is the premise drug in the form of isopropyl ester. It is absorbed through the cornea and hydrolyzed into a biologically active free acid by the action of esterase to increase the scleral pigment efflux of aqueous humor to reduce intraocular pressure. This product can also make glaucoma patients usually block the eyeball trabecular structure screen.
Carcinogenic, mutagenic, and survival: This product has no mutagenic effects in bacteria, mouse lymphoma cells, and micronucleus tests.
In the human lymphocyte experiment, the product has chromosome aberrations.
Mice and rats were orally administered at 170 μg/kg/d (about 2800 times the maximum dose for human use) for 20-24 months, and no carcinogenic effect was observed.
In vivo and in vitro studies in rats have shown that this product does not interfere with DNA synthesis. Animal studies have shown that this product has no effect on the survival of males and females.
Teratogenic effect: The rabbit pregnancy test showed that when the amount of this product was 80 times higher than the maximum dosage of the human body, 4/16 female rabbits had a stillbirth. It is estimated that the dose of rabbit embryo-free toxicity is 15 times of the maximum dosage of the human body. This study was not performed in pregnant women due to lack of appropriate controls. Pregnancy women should weigh the pros and cons when using this product.
【Pharmacokinetics】
This product is hydrolyzed into free acid in the cornea. This free acid diffuses out of the cornea and enters the aqueous humor. It can reach the peak of blood in about 2 hours. After 3~4h, the intraocular pressure began to decrease, and the maximum decrease was reached at 8~12h, and the intraocular pressure did not increase after 24h. The drug is excreted as it flows out and has a half-life of about 2 hours. Systemic absorption is produced through the conjunctiva or mucosa, and the absorbed drug is mainly excreted in the blood circulation system, and is mainly excreted in the urine after being metabolized by the liver.
【Storage】Store at 2 °C to 8 °C before opening, and keep it away from light. It can be stored at room temperature below 25 °C after opening.
【Packaging】 Plastic bottle, 1 bottle / box.
【Validity period】tentatively for 24 months.
【Executive Standards】National Food and Drug Administration National Drug Standard YBH09872004.
【Approval No.】National Drug Standard H20044234.
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 0539-8336337 (Quality Pipe Department)
Fax number: 0539-8336029 (Sales) 0539-8336338 (Quality Pipe Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
