Montelukast Sodium Tablets
DescriptionFull Analysis capability
Thin Film Coating Lab
Montelukast Sodium Tablets
Indication
This product is intended for the prevention and long-term treatment of asthma in adults aged 15 years and older, including prevention of asthma symptoms during the day and night, treatment of asthma patients who are sensitive to aspirin, and prevention of exercise-induced bronchoconstriction. This product is suitable for alleviating the symptoms caused by allergic rhinitis (seasonal allergic rhinitis and perennial allergic rhinitis in adults aged 15 and over).
Clinical pharmacology
Montelukast sodium is a potent oral preparation that significantly improves asthma inflammation. Biochemical and pharmacological bioassays show that montelukast has a high affinity and selectivity for type I cysteinyl leukotriene (CysLT1) receptors (with other pharmacologically important airway receptors) Such as prostaglandins, cholinergic and β-adrenergic receptors). Montelukast can effectively inhibit the physiological effects produced by the binding of LTC4, LTD4 and LTE4 to the CysLT1 receptor without any receptor agonistic activity. Current research suggests that montelukast does not antagonize the CysLT2 receptor.
Dosage
Patients 15 years of age and older with asthma and/or allergic rhinitis once daily, one tablet at a time (10 mg). Asthmatic patients should take it before going to bed. Patients with seasonal allergic rhinitis can take their medication when needed according to their own conditions. Patients with both asthma and allergic rhinitis should be given once a night.
Formulation
tablet
specification
10mg (according to montelukast).
Instruction manual
Approval date: May 13, 2008
Date of revision: July 29, 2008
January 06, 2014 2016-4-21
Montelukast sodium tablets instructions
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: Montelukast sodium tablets
English name: Montelukast Sodium Tablets
Pinyin: Menglusitena Pian
【Ingredients】 The main ingredient of this product is montelukast sodium.
Chemical name: [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinoline)vinyl]phenyl]-3-[2-(1-hydroxy) Sodium 1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetate.
Chemical Structure:
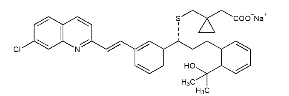
Molecular formula: C35H35ClNNaO3S Molecular weight: 608.17
【Properties】This product is a yellow film-coated tablet, which is white or off-white after removal of the film coat.
【indications】
This product is intended for the prevention and long-term treatment of asthma in adults aged 15 years and older, including prevention of asthma symptoms during the day and night, treatment of asthma patients who are sensitive to aspirin, and prevention of exercise-induced bronchoconstriction.
This product is suitable for alleviating the symptoms caused by allergic rhinitis (seasonal allergic rhinitis and perennial allergic rhinitis in adults aged 15 and over).
【Specification】10mg (according to Montelust).
【Dosage】
Patients 15 years of age and older with asthma and/or allergic rhinitis once daily, one tablet at a time (10 mg). Asthmatic patients should take it before going to bed. Patients with seasonal allergic rhinitis can take their medication when needed according to their own conditions.
Patients with both asthma and allergic rhinitis should be given once a night.
General advice
The therapeutic effect was evaluated by the asthma control index, and the efficacy of the product appeared within one day of the drug administration. This product can be taken with food or served separately. Patients should be advised to take it regardless of asthma control or deterioration.
Older patients, patients with renal insufficiency, patients with mild to moderate liver damage, and patients of different genders do not need to adjust the dose.
Relationship between montelukast sodium tablets and other asthma treatment drugs
This product can be added to the patient's existing treatment plan.
Reduce the dose of the combined medication:
Bronchodilator:
In asthma patients who cannot be effectively controlled with bronchodilator alone, this product can be added to the treatment regimen. Once there is a clinical response (usually after the first dose), the dose of bronchodilator can be reduced according to the patient's tolerance. .
Inhaled corticosteroids:
For patients with asthma who are treated with inhaled corticosteroids, the dose of corticosteroids can be appropriately reduced according to the patient's tolerance. It should be gradually reduced under medical supervision. Some patients can gradually reduce the amount until inhaled corticosteroids are completely discontinued. However, this product should not be used to suddenly replace inhaled corticosteroids or as directed by your doctor.
【Adverse reactions】
This product is generally well tolerated, with mild side effects and usually does not require termination of treatment. The overall incidence of adverse reactions was similar to placebo.
Asthma patients aged 15 and over
Clinical studies have been conducted in approximately 2,600 adult asthma patients aged 15 years and older to evaluate the safety of this product. In two similarly designed, placebo-controlled, 12-week clinical studies, the drug-related incidence in the treatment group was ≥1% and the adverse events in the placebo group were abdominal pain and headache. However, the incidence of these adverse events did not differ significantly between the two groups.
In clinical studies, 544 patients have been accumulating with this product for at least 6 months, 253 patients for 1 year, and 21 patients for 2 years. With the prolongation of treatment time with this product, the occurrence of adverse events did not change.
Seasonal allergic rhinitis patients aged 15 and over
Clinical studies have been conducted in 2199 adult patients with seasonal allergic rhinitis aged 15 years and older to evaluate the safety of this product. Taking this product once a day or night is well tolerated, and the incidence of adverse reactions is similar to taking a placebo. In a placebo-controlled clinical study, no drug-related incidence was found to be ≥1% in the treatment group and higher than in the placebo group. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with the 2-week clinical study. In all clinical studies, the incidence of lethargy was similar to that of the placebo group.
Perennial allergic rhinitis patients aged 15 and over
Two six-week, placebo-controlled clinical studies have been conducted in 3,235 adult patients with perennial allergic rhinitis aged 15 years and older to evaluate the safety of this product. The daily use of this product was well tolerated, and the incidence of adverse reactions was similar to that of the placebo group, and was consistent with the clinical study of seasonal allergic rhinitis. In both clinical studies, the incidence of adverse events was less than 1% in the treatment group and no drug-related events were found, which was higher than the adverse events in the placebo group. The incidence of lethargy was similar to that of the placebo group.
Combined analysis of clinical practice
A pooled analysis of 41 placebo-controlled clinical studies (35 studies for patients aged 15 years and older; 6 studies for children aged 6-14 years) was performed using an effective suicidal behavioral assessment. Among 9929 patients taking this product and 7780 patients taking placebo, one patient with suicidal ideation took this product. There were no preparatory actions to complete suicide, suicide attempts or suicidal behavior in any group.
For 46 placebo-controlled clinical studies (35 studies for eloquent 11-year-old studies of children aged 15 years and older for children between 3 months and 14 years of age), an independent combined analysis was performed to evaluate behavior-related adverse events. The incidence of behavior-related adverse events was 2.73% and 2.27% in patients who took this product at 11,673 and in 8827 patients who received placebo; the odds ratio was 1.12 (95 CI [0.93; 1.36]).
Clinical trials included in these combined analyses did not have specific design suicide rates or behavior-related adverse events.
Post-marketing experience
This product has the following adverse reaction reports after its use:
Infection and infection: upper respiratory tract infection.
Blood and lymphatic system disorders: increased bleeding tendency.
Immune system disorders: hypersensitivity reactions including allergic reactions, very rare hepatic eosinophil infiltration.
Mental system disorders: including aggressive behavior or hostile excitement, anxiety, depression, loss of direction perception, inattention, night dreams, hallucinations, insomnia, memory impairment, hyperkinesia (including irritability, restlessness) And tremor), sleepwalking, suicidal thoughts and behaviors (suicide).
Nervous system disorders: dizziness, lethargy, paresthesia/tactile sensation, and very rare seizures.
Heart disorder: palpitations.
Respiratory, thoracic and mediastinal disorders: nasal discharge; pulmonary eosinophilia.
Gastrointestinal disorders: diarrhea, indigestion, nausea, vomiting.
Hepatobiliary disorders: elevated ALT and AST, very rare hepatitis (including cholestatic, hepatocytes and mixed liver damage).
Skin and subcutaneous tissue disorders: angioedema, contusion, erythema multiforme, nodular erythema, itching, rash, urticaria.
Musculoskeletal and connective tissue disorders: joint pain, myalgia including muscle spasms.
Other disorders and site of administration: weakness / fatigue, edema, fever.
【Contraindications】 It is forbidden to be allergic to any of the ingredients in this product.
【Precautions】
The efficacy of oral administration of this product in the treatment of acute asthma attacks has not been determined. Therefore, it should not be used to treat acute asthma attacks.
Although the inhaled glucocorticoid dose can be gradually reduced under the guidance of a physician, this product is not used to suddenly replace inhaled or oral glucocorticoids.
Patients taking this product have reported a neurological event (see Adverse Reactions). Since other factors may also cause these events, it is not possible to confirm whether it is related to this product. The doctor should discuss these adverse events with the patient and/or caregiver. The patient and/or caregiver should be informed that the doctor should be notified if these conditions occur.
In patients receiving anti-asthmatic medications, including leukotriene receptor antagonists, when reducing the dose of systemic glucocorticoids, very few cases occur in one or more of the following cases: eosinophilia, vascular Rash, worsening of lung symptoms, cardiac complications and/or neuropathy (sometimes diagnosed as Churg-Strauss syndrome - a systemic eosinophilic vasculitis). Although the causal relationship between these conditions and leukotriene receptor antagonists has not been determined, it is recommended that attention should be paid to appropriate clinical monitoring when reducing the systemic glucocorticoid dose in patients receiving this product.
【Pregnant women and lactating women】
For pregnant women without research data, pregnant women should avoid taking this product unless they are explicitly required to take the drug.
Experience after global marketing shows that there are rare reports of congenital limb defects in newborns after using this product during pregnancy. Most of these women also use other asthma treatments during pregnancy. The causal relationship between the use of this product and these events has not been established.
It is not clear whether this product can be secreted from milk. Since many drugs can be secreted from milk, breast-feeding women should use this product with caution.
【Children's medication】
For patients aged 15 to 18, see [Usage and Dosage]. Studies have shown that this product does not affect the growth rate of children. This product specification is not suitable for children under 15 years of age.
【Geriatric Use】 In clinical studies, there is no age difference in the effectiveness and safety of this product.
【medicine interactions】
This product can be combined with other drugs commonly used for the prevention and long-term treatment of asthma and for the treatment of allergic rhinitis. In drug interaction studies, the recommended dose of this product does not have clinically significant pharmacokinetic effects on the following drugs: theophylline, prednisone, prednisolone, oral contraceptives (ethinyl estradiol / norethisterone) 35/1), terfenadine, digoxin and warfarin.
In patients with phenobarbital combined, the area under the plasma concentration-time curve (AUC) of montelukast was reduced by approximately 40%. However, it is not recommended to adjust the dosage of this product.
In vitro tests have shown that montelukast is an inhibitor of CYP2C8. However, a clinical study of the drug interactions between montelukast and rosiglitazone, a typical probe substrate that is primarily metabolized by CYP2C8, suggests that montelukast does not inhibit CYP2C8 in vivo. Therefore, it is believed that montelukast does not affect drugs that are metabolized by this enzyme (eg, paclitaxel, rosiglitazone, repaglinide).
【Drug overdose】
There is no specific information on the overdose of this product in clinical treatment. In the study of chronic asthma, adult patients used a dose of up to 200 mg per day for 22 weeks, and the dose used in the short-term study was as high as 900 mg per day for about 1 week. No clinically significant adverse events occurred.
There have been reports of acute drug overdose after marketing and clinical studies using this product. This includes reports of up to 1000 mg doses for adults and children. Clinical and laboratory findings consistently show their safety in both adult and pediatric patients. In most reports of drug overdose, there were no adverse events. The most common adverse events are consistent with safety features, including abdominal pain, lethargy, thirst, headache, vomiting, and hyperkinesia.
It is not clear whether this product can be removed by peritoneal or hemodialysis.
【Pharmacology and Toxicology】
Pharmacology
Montelukast sodium is a potent oral preparation that significantly improves asthma inflammation. Biochemical and pharmacological bioassays show that montelukast has a high affinity and selectivity for type I cysteinyl leukotriene (CysLT1) receptors (with other pharmacologically important airway receptors) Such as prostaglandins, cholinergic and β-adrenergic receptors). Montelukast can effectively inhibit the physiological effects produced by the binding of LTC4, LTD4 and LTE4 to the CysLT1 receptor without any receptor agonistic activity. Current research suggests that montelukast does not antagonize the CysLT2 receptor.
toxicology
Repeated dosing toxicity: In the 14-week toxicity test in monkeys and mice, monkeys and rats showed good tolerance to montelukast sodium. No toxicity effects were found on the toxicological indicators when using at least a human recommended dose of at least 125 times the recommended dose of montelukast sodium to any of the animals tested.
Genotoxicity: Montelukast sodium has not been found to be genotoxic and mutagenic. In the in vitro microbial mutation test and the V-79 mammalian cell mutation test, montelukast sodium was negative in the presence or absence of metabolic activity. In the rat liver cell alkaline elution test and the Chinese hamster ovary cell chromosome aberration test in vitro, there was no genotoxic effect with or without the microsomal enzyme activity system. Similarly, when male or female mice were orally administered up to 1200 mg/kg (3600 mg/m2) (6000 times the recommended daily dose for adults, 50 kg of adult weight) of montelukast sodium, no chromosomal abnormalities were induced in bone marrow cells. The role.
Reproductive toxicity: In male rats, the dose of oral montelukast sodium was as high as 800 mg/kg/day and the oral dose of female rats was as high as 100 mg/kg/day. No effect on fertility and fertility was found. These doses are respectively 4000 and 500 times higher than the recommended dose for adults (based on adult weight 50 kg).
In the developmental toxicity study, no adverse effects associated with treatment were found in rats when the dose was as high as 400 mg/kg/day and when the dose of montelukast sodium was as high as 100 mg/kg/day in rabbits. There was indeed a case in which the fetus was exposed to montelukast sodium in rats and rabbits, and montelukast sodium was clearly detected in the milk of the lactating rats.
Carcinogenicity: In the study of oral doses of up to 200 mg/kg/day for 106 weeks in rats and oral doses of up to 100 mg/kg/day for 92 weeks in mice, no clinical efficacy of montelukast sodium was found to be carcinogenic. These doses are equivalent to 1000 times and 500 times the recommended dose for adults (based on adult weight 50 kg).
【Pharmacokinetics】
absorb
Oral absorption of montelukast is rapid and complete. After taking 10 mg of the film-coated tablets on an empty stomach, the plasma concentration reached a peak concentration (Cmax) at 3 hours (Tmax). The average oral bioavailability was 64%. Ordinary diet has no effect on oral bioavailability and Cmax. Clinical studies have shown that montelukast sodium, which takes 10 mg of film-coated tablets at any time after eating, is safe and effective.
distributed
More than 99% of montelukast sodium binds to plasma proteins. The steady state distribution volume of montelukast is 8 to 11 liters on average. Studies of isotope-labeled montelukast in rats have shown that only a very small amount of montelukast passes through the blood-brain barrier. Moreover, the amount of radiolabel in all other tissues was extremely small 24 hours after administration.
metabolism
Montelukast is almost completely metabolized. In the study using therapeutic doses, metabolites of montelukast were not detected in plasma in the steady state of adults and children.
Studies using human liver microsomes in vitro have shown that cytochrome P450 3A4 and 2C9 are involved in the metabolism of montelukast. According to further studies of human liver microsomes in vitro, the plasma concentration of the montelukast treatment dose does not inhibit cytochrome P450 3A4, 2C9, 1A2, 2A6, 2C19 or 2D6.
excretion
The mean plasma clearance of montelukast in healthy adults was 45 ml/min. After oral isotope-labeled montelukast, 86% of radioactivity was detected in feces collected over the next 5 days, and the amount measured in urine was <0.2%. In combination with the oral bioavailability of montelukast, montelukast and its metabolites are almost exclusively excreted via bile.
Many studies conducted in healthy youth have shown that the mean plasma half-life of montelukast is 2.7 to 5.5 hours. The pharmacokinetics of montelukast approximates a linear relationship in the range of oral doses up to 50 mg. There was no difference in the pharmacokinetics of taking montelukast in the morning and at night. Taking 10 mg of montelukast once a day, only a small amount of the original drug accumulated in the plasma (~14%).
Special patient
There is no need to adjust the dose for elderly, patients with renal insufficiency or patients with mild to moderate hepatic insufficiency. Clinical data on montelukast were not available in patients with severe hepatic insufficiency (Child-Pugh score >9).
【Storage】 Protect from light, seal, and store in a cool place (not more than 20 ° C).
【Packing】Double aluminum packaging. 6 pieces / box, 12 pieces / box, 18 pieces / box, 24 pieces / box.
【Validity Period】36 months.
【Executive Standards】National Food and Drug Administration Standard YBH04912008
【Approval No.】 Guoyao Zhunzi H20083372
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 8336337 (Quality Management Department)
Fax number: 0539-8336029 (Sales) 8336338 (Quality Management Department)
Website: www.lunan.com.cn
24-hour customer service hotline: 400-0539-310
