Rosuvastatin Calcium Tablets
DescriptionFull Analysis capability
Thin Film Coating Lab
Rosuvastatin Calcium Tablets
Indication
This product is suitable for primary hypercholesterolemia (type IIa, including heterozygous familial hypercholesterolemia) that is not adequately controlled by dietary control and other non-pharmacological treatments (eg, exercise therapy, weight loss) or Mixed dyslipidemia (type IIb). This product is also suitable for patients with homozygous familial hypercholesterolemia, as an adjunct to diet control and other lipid-lowering measures (such as LDL removal therapy), or when these methods are not applicable.
Clinical pharmacology
Rosuvastatin is a selective, competitive HMG-CoA reductase inhibitor. HMG-CoA reductase is the rate-limiting enzyme in the conversion of 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, which is a precursor of cholesterol. Animal experiments and cell culture test results show that rosuvastatin is highly and highly selective in the liver, and the liver is a target organ for lowering cholesterol. In vivo and in vitro tests have shown that rosuvastatin can increase the number of hepatic LDL receptors on the cell surface, thereby enhancing LDL uptake and catabolism, and inhibiting liver VLDL synthesis, thereby reducing the total number of VLDL and LDL particles. For homozygous and heterozygous familial hypercholesterolemia patients, non-familial hypercholesterolemia patients, mixed dyslipidemia patients, rosuvastatin can reduce total cholesterol, LDL-C, ApoB, non-HDL-C levels. Rosuvastatin also lowers TG and raises HDL-C levels. For patients with hypertriglyceridemia alone, rosuvastatin reduced total cholesterol, LDL-C, VLDL-C, ApoB, non-HDL-C, TG levels, and elevated HDL-C levels. The effects of rosuvastatin on cardiovascular morbidity and mortality have not been determined.
Dosage
Patients should be given a standard cholesterol-lowering diet control prior to treatment initiation and maintain dietary control during treatment. The use of this product should follow the principle of individualization, taking into account the individual's individual cholesterol level, expected cardiovascular risk and the potential risk of adverse reactions... (see instructions)
Formulation
tablet
specification
(1) 5 mg; (2) 10 mg; (3) 20 mg
Instruction manual
Approval date: May 4, 2008
Date of revision: March 13, 2012
December 10, 2013
Rosuvastatin calcium tablets instructions
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: rosuvastatin calcium tablets
English name: Rosuvastatin Calcium Tablets
Pinyin: Ruishufatatinggai Pian
【Ingredients】 The main ingredient of this product is rosuvastatin calcium.
Chemical name: bis-[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-pyrimidin-5-yl]( 3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt (2:1)
Chemical Structure:
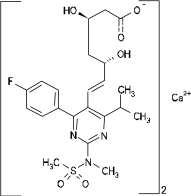
Molecular formula: (C22H27FN3O6S) 2Ca Molecular weight: 1001.15
【Properties】 This product is a film-coated tablet, which is white or off-white after removal of the coating.
【indications】
This product is suitable for primary hypercholesterolemia (type IIa, including heterozygous familial hypercholesterolemia) that is not adequately controlled by dietary control and other non-pharmacological treatments (eg, exercise therapy, weight loss) or Mixed dyslipidemia (type IIb).
This product is also suitable for patients with homozygous familial hypercholesterolemia, as an adjunct to diet control and other lipid-lowering measures (such as LDL removal therapy), or when these methods are not applicable.
【Specifications】 according to rosuvastatin (C22H27FN3O6S)
(1) 5 mg; (2) 10 mg; (3) 20 mg
【Dosage】
Patients should be given a standard cholesterol-lowering diet control prior to treatment initiation and maintain dietary control during treatment. The use of this product should follow the principle of individualization, taking into account the individual's individual cholesterol level, the expected cardiovascular risk and the potential risk of adverse reactions.
Oral. This product is usually used at a starting dose of 5mg once a day. The choice of starting dose should take into account the individual's individual cholesterol levels, the expected cardiovascular risk, and the potential risk of adverse reactions. For those patients who need to more effectively lower LDL-C (LDL-C), 10 mg once a day can be considered as a starting dose, which can control blood lipid levels in most patients. If necessary, the dose can be adjusted to a higher level after 4 weeks of treatment. The maximum daily dose of this product is 20mg.
This product can be administered at any time of the day and can be taken while eating or on an empty stomach.
Patients with renal insufficiency medication
Patients with mild and moderate renal impairment do not need to adjust the dose. All doses of this product are contraindicated in patients with severe renal impairment.
Drug use in patients with liver damage
In subjects with a Child-Pugh score no higher than 7, systemic exposure to rosuvastatin was not elevated. In subjects with Child-Pugh scores of 8 and 9, an increase in systemic exposure was observed. In these patients, an assessment of renal function should be considered. There is no experience in using this product in patients with a Child-Pugh score of over 9. This product is contraindicated in patients with active liver disease.
Race
An increase in systemic exposure in Asian subjects has been observed. This factor should be considered when determining the dose of a patient with Asian descent.
【Adverse reactions】
The adverse reactions seen in this product are usually mild and transient. In foreign controlled clinical trials, less than 4% of patients withdrew from the trial due to adverse events.
The frequency of adverse events is arranged in the following order: common (incidence > 1/100, <1/10); rare (>1/1000, <1/100); rare (>1/10000, <1/1000); Very rare (<1/10000).
Immune system abnormality
Rare: Allergic reactions, including angioedema.
Nervous system abnormality
Common: headache, dizziness
Abnormal gastrointestinal tract
Common: constipation, nausea, abdominal pain
Abnormal skin and subcutaneous tissue
Rare: itching, rash and urticaria
Skeletal muscle, joint and bone abnormalities
Common: myalgia
Rare: myopathy and rhabdomyolysis
Abnormal body
Common: powerless
Like other HMG-CoA reductase inhibitors, the incidence of adverse reactions in this product tends to increase with increasing dose.
Effects on the kidney: Proteinuria (test strip test) was observed in patients receiving this product, and most of the protein was derived from the renal tubules. Approximately 1% of patients had no or minimal increase in proteinuria to ++ or more during certain periods of 10 mg and 20 mg of treatment, which was approximately 3% in patients receiving 40 mg of treatment. In the 20 mg dose treatment, a slight increase in proteinuria from no or slightly increased to + was observed. In most cases, proteinuria is automatically reduced or disappeared after continued treatment.
Effects on skeletal muscle: There are reports of effects on skeletal muscle in patients receiving various doses of this product, such as myalgia, myopathy, and rare rhabdomyolysis, especially in patients with doses greater than 20 mg. .
Elevated levels of creatine kinase (CK) were observed to be dose-related in patients taking this product; most cases were mild, asymptomatic, and transient. If CK levels are elevated (>5 x ULN), treatment should be discontinued.
Effects on the liver: As with other HMG-CoA reductase inhibitors, dose-related elevations of transaminases were observed in a small number of patients taking this product; most cases were mild, asymptomatic, and transient.
Post-marketing experience: Post-marketing monitoring of statins has reports of hyperglycemia, impaired glucose tolerance, elevated glycated hemoglobin levels, new-onset diabetes, worsening glycemic control, and some statins have reports of hypoglycemia.
There are rare cases of cognitive impairment in the post-marketing surveillance of statins, which are memory loss, memory loss, confusion, etc., mostly non-severe, reversible reactions, which can be recovered after stopping the drug.
In addition to the above reactions, the following adverse events were reported during the post-marketing use of this product:
Hepatobiliary diseases:
Very rare: jaundice, hepatitis;
Rare: elevated liver transaminases.
Musculoskeletal disorders:
Rare: joint pain
Nervous system disease:
Very rare: polyneuropathy
【taboo】
This product is forbidden to:
● Those who are allergic to rosuvastatin or any of the ingredients in this product.
● Patients with active liver disease, including patients with unexplained elevated serum transaminases and any elevated serum transaminases that exceed the 3-fold upper limit of normal (ULN).
● Patients with severe renal impairment (creatinine clearance <30 ml/min).
● Patients with myopathy.
● Patients who use cyclosporine at the same time.
● Women who are pregnant, breastfeeding, and who are likely to become pregnant without proper contraception.
【Precautions】
Effect on the kidney
In high doses, especially in patients treated with 40 mg, proteinuria (test strip test) was observed. Most of the protein was derived from the renal tubules. In most cases, proteinuria was transient or intermittent.
Effect on skeletal muscle
There have been reports of effects on skeletal muscle in patients receiving various doses of this product, such as myalgia, myopathy, and rare rhabdomyolysis, especially in patients with doses greater than 20 mg.
Creatine kinase assay
Creatine kinase (CK) should not be detected after strenuous exercise or in the presence of plausible factors that cause CK elevation, which would confuse the interpretation of the results. If the CK base value is significantly increased (>5×ULN), the test should be confirmed within 5-7 days. If repeated testing confirms that the patient's CK baseline value is >5 x ULN, treatment cannot begin.
Before treatment
As with other HMG-CoA reductase inhibitors, patients with myopathy/rhabdomin susceptibility should be cautious when using this product.
These factors include:
● Renal impairment
● Hypothyroidism
● Hereditary muscle disease in my or family history
● Previous history of muscle toxicity of other HMG-CoA reductase inhibitors or fibrates
● Alcohol abuse
● Age > 70 years old
● Possible increase in blood concentration
● Use the Bate class at the same time
For these patients, the relationship between the possible benefits of treatment and the potential risks should be considered, and clinical monitoring is recommended. If the patient's CK baseline value is significantly elevated (>5 x ULN), treatment should not begin.
In treatment
Patients should be asked to immediately report muscle pain, weakness or spasms of unknown cause, especially if they are accompanied by discomfort and fever. These patients should be tested for CK levels. Treatment should be discontinued if the CK value is significantly elevated (>5 x ULN) or if the muscle symptoms are severe and cause discomfort throughout the day (even if CK ≤ 5 x ULN). If symptoms are resolved and CK levels return to normal, consider re-administering the product or switching to the lowest dose of other HMG-CoA reductase inhibitors and observe closely.
Regular testing of CK levels in asymptomatic patients is not required.
In clinical studies, there is no evidence that the effects of drugs on skeletal muscle are increased in a small number of patients who use this product and other treatments. However, it has been found that other HMG-CoA reductase inhibitors and beryllic acid derivatives (including gemfibrozil), cyclosporine, nicotinic acid, azole antifungal agents, protease inhibitors or macrolide antibiotics The incidence of myositis and myopathy increased in patients who were combined. Gemfibrozil is used in combination with some HMG-CoA reductase inhibitors to increase the risk of myopathy. Therefore, this product is not recommended for use with Gemfibrozil. Care should be taken to weigh the benefits of this product in combination with fibrates or niacin to further improve lipid levels and the potential risks of this combination.
Any acute or severe renal failure associated with myopathy (such as sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte abnormalities, or uncontrolled epilepsy) This product is not available for patients.
Impact on the liver
As with other HMG-CoA reductase inhibitors, this product should be used with caution in patients with excessive alcohol and/or liver disease. It is recommended to perform liver function tests before starting treatment and at 3 months after starting. If serum transaminase rises more than 3 times the upper limit of normal, this product should be discontinued or reduced.
For hypercholesterolemia secondary to hypothyroidism or nephrotic syndrome, the primary disease should be treated before starting treatment with this product.
Race
Pharmacokinetic studies have shown that Asian subjects have higher exposures than Caucasians.
Impact on driving vehicles and manipulating machines
Research to determine the impact of this product on driving vehicles and manipulating machines has not been conducted. However, depending on the pharmacodynamic properties, this product is unlikely to affect these capabilities. When driving a vehicle and manipulating the machine, it should be considered that vertigo may occur during treatment.
【Pregnant women and lactating women】
This product is contraindicated in pregnant women and lactating women.
Women who are likely to become pregnant should take appropriate contraceptive measures.
Since cholesterol and other cholesterol biosynthesis products are important for embryo development, the risk of inhibition from HMG-CoA reductase exceeds the benefit of treatment for pregnant women. Animal studies provide evidence of limited reproductive toxicity. If the patient is pregnant during the use of this product, treatment should be discontinued immediately. Rosuvastatin can be secreted into rat milk. There is no information on the secretion of rosuvastatin into human milk.
【Children's medication】
The safety and efficacy of this product in children has not been established. The experience of pediatric use is limited to a small number of children (age ≥ 8 years) with homozygous familial hypercholesterolemia. Therefore, it is not currently recommended for pediatrics to use this product.
【Geriatric Use】
There is no need to adjust the dose.
【medicine interactions】
Cyclosporine: When used in combination with cyclosporine, the AUC of rosuvastatin was 7 times higher than that observed in healthy volunteers (compared to the same dose of this product). The combined use does not affect the plasma concentration of cyclosporine.
Vitamin K Antagonists: As with other HMG-CoA reductase inhibitors, starting a patient with a vitamin K antagonist (eg warfarin) or gradually increasing the dose may result in an increase in INR. Disabling this product or gradually reducing the dose of this product can result in a decrease in INR. In this case, it is necessary to properly detect the INR.
Gemfibrozil and other lipid-lowering products: This product can be used in combination with gemfibrozil to increase the Cmax and AUC of rosuvastatin by a factor of two.
Based on data from specialized interaction studies, this product is expected to interact with fenofibrate without pharmacokinetics, but pharmacodynamic interactions may occur.
Gemfibrozil, fenofibrate, other fibrates and lipid-lowering doses (≥1g/day) of niacin combined with HMG-CoA reductase inhibitors increase the risk of myopathy, which may be due to their separate It can cause myopathy when administered.
Antacids: Simultaneous administration of this product and an antacid suspension containing magnesium aluminum hydroxide reduced the plasma concentration of rosuvastatin by approximately 50%. If the antacid is given 2 hours after taking this product, the effect can be alleviated. The clinical significance of this drug interaction has not been studied.
Erythromycin: This product combined with erythromycin resulted in a 20% decrease in AUC (0-t) and a 30% decrease in Cmax of rosuvastatin. This interaction may be due to an increase in gastrointestinal motility caused by erythromycin.
Oral contraceptive/hormone replacement therapy (HRT): The use of this product and oral contraceptives increased the AUC of ethinyl estradiol and norgestrel by 26% and 34%, respectively. These increases in blood concentration should be considered when selecting an oral contraceptive dose. There are no pharmacokinetic data for subjects who use both this product and HRT, so similar interactions cannot be ruled out. However, in clinical trials, this combination is widely available and well tolerated by patients.
Other drugs: Based on data from specialized drug interaction studies, it is estimated that there is no clinically relevant interaction between this product and digoxin.
Cytochrome P450 enzymes: Both in vitro and in vivo studies have shown that rosuvastatin is neither an inhibitor of cellular P450 isoenzymes nor an enzyme inducer. In addition, rosuvastatin is a weak substrate for these enzymes. There is no clinically relevant interaction between rosuvastatin and fluconazole (an inhibitor of CYP 2CP and CYP 3A4) or ketoconazole (an inhibitor of CYP 2A6 and CYP 3A4). In combination with itraconazole (an inhibitor of CYP 3A4), the AUC of rosuvastatin increased by 28%, and this increase was not considered clinically significant. Therefore, it is estimated that there is no drug interaction caused by cytochrome P450-mediated metabolism.
Protease inhibitors: Although the mechanism of drug interactions is not clear, simultaneous administration of protease inhibitors may greatly increase the exposure of rosuvastatin. In the pharmacokinetic study, healthy volunteers taking a combination of 20 mg of this product and two protease inhibitors (400 mg lopinavir/100 mg ritonavir) showed the steady-state AUC of rosuvastatin ( 0-24) and Cmax increased by about 2 times and 5 times, respectively. Therefore, it is not recommended to use this product in HIV patients who are treated with protease inhibitors.
【Drug overdose】
There is no special treatment when this product is overdose. Once an overdose occurs, symptomatic treatment should be given and supportive measures should be taken when needed. Liver function and CK levels should be monitored. Hemodialysis may not have a significant effect.
【Pharmacology and Toxicology】
Pharmacological action
Rosuvastatin is a selective, competitive HMG-CoA reductase inhibitor. HMG-CoA reductase is the rate-limiting enzyme in the conversion of 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, which is a precursor of cholesterol. Animal experiments and cell culture test results show that rosuvastatin is highly and highly selective in the liver, and the liver is a target organ for lowering cholesterol. In vivo and in vitro tests have shown that rosuvastatin can increase the number of hepatic LDL receptors on the cell surface, thereby enhancing LDL uptake and catabolism, and inhibiting liver VLDL synthesis, thereby reducing the total number of VLDL and LDL particles.
For homozygous and heterozygous familial hypercholesterolemia patients, non-familial hypercholesterolemia patients, mixed dyslipidemia patients, rosuvastatin can reduce total cholesterol, LDL-C, ApoB, non-HDL-C levels. Rosuvastatin also lowers TG and raises HDL-C levels. For patients with hypertriglyceridemia alone, rosuvastatin reduced total cholesterol, LDL-C, VLDL-C, ApoB, non-HDL-C, TG levels, and elevated HDL-C levels. The effects of rosuvastatin on cardiovascular morbidity and mortality have not been determined.
Toxicological research
Central nervous system toxicity
In the canine test of several similar drugs, CNS vascular injury was observed, and perivascular hemorrhage, edema, and perivascular mononuclear cell infiltration were observed. A drug similar in structure to this class of drugs exhibits dose-dependent optic nerve degeneration (retina-knee body fiber Wallerian degeneration) at a dose that is 30 times higher than the mean concentration of the human maximum recommended dose in dogs.
One female dog was orally administered with rosuvastatin 90 mg/kg/day (calculated according to AUC, systemic exposure was equivalent to 100 times of human 40 mg/day exposure), and euthanized on the 24th day due to sudden death, showing choroid plexus Edema, hemorrhage, partial necrosis. The dog was given rosuvastatin 6 mg/kg/day by mouth (calculated according to AUC, the systemic exposure was equivalent to 20 times of the human 40 mg/day exposure), and the cornea was cloudy for 52 weeks. Canine oral administration of rosuvastatin 30 mg / kg / day (according to AUC, systemic exposure is equivalent to 60 times the human 40mg / day exposure), for 12 weeks, cataracts can be seen. Canine oral administration of rosuvastatin 90 mg / kg / day (according to AUC, systemic exposure is equivalent to 100 times the human 40mg / day exposure), for 4 weeks, visible retinal dysplasia and retinal detachment. Dogs were administered continuously for 1 year at doses ≤ 30 mg/kg/day (calculated by AUC, systemic exposure equivalent to 60 times exposure to human 40 mg/day), and no effect on the retina was observed.
Genotoxicity
The results of rosuvastatin in the Ames test, mouse lymphoma test, CHL cell chromosome aberration test, and mouse micronucleus test were all negative.
Reproductive toxicity
In the rat fertility test, male rats were orally administered 5, 15, 50 mg/kg/day from the 9th week before mating to the mating period, and 2 weeks before the mating of the female rats, at the highest dose (according to AUC). It is estimated that the systemic exposure is equivalent to 10 times the exposure of human 40 mg/day. There is no adverse effect on fertility. The dog was orally administered with rosuvastatin 30 mg/kg/day for 1 month, and the spermatophore (Spermatidic giant cell) was observed in the testis. Monkeys were given rosuvastatin 30 mg/kg/day for 6 months, and the macrophage and vas deferens were vacuolated. The above doses of dogs and monkeys were estimated to be 20 and 10 times the body surface area of 40 mg/day, respectively. Similar phenomena can be seen with similar drugs.
Female rats were orally administered 5, 15, 50 mg/kg/day 7 days after mating, and high-dose group (calculated as AUC, systemic exposure was equivalent to 10 times of human 40 mg/day exposure). Delayed ossification.
Rats were orally administered 2, 10, 50 mg/kg/day from the 7th day of pregnancy to the 21st day of lactation (weaning), and the high dose group (calculated by body surface area, 12 times or more of human 40mg/day) The survival rate of the child is reduced. Rabbits were orally administered 0.3, 1, 3 mg/kg/day from the 6th day of pregnancy to the 18th day of lactation (weaning) (calculated by body surface area, equivalent to 40mg/day), showing a decrease in fetal survival rate, maternal animal death. The dose of rosuvastatin was ≤25 mg/kg/day in rats and ≤3 mg/kg/day in rabbits. No teratogenicity was found (calculated as AUC and body surface area, respectively, comparable to human exposure at 40 mg/day).
Carcinogenicity
In the 104-week carcinogenicity test in rats, the oral dose was 2, 20, 60, 80 mg/kg/day. 80mg/kg/day (calculated by AUC, systemic exposure is equivalent to 20 times of human 40mg/day exposure). The incidence of uterine polyps was significantly increased in the female group of the dose group, and the incidence was not increased at low doses.
In the 107-week carcinogenicity test of mice, the oral dose was 10, 60, 200 mg/kg/day. 200mg/kg/day (calculated by AUC, systemic exposure is equivalent to 20 times of human 40mg/day exposure). The dose group showed an increase in hepatocellular adenoma/carcinogenesis, and no increase in low dose.
【Pharmacokinetics】
The pharmacokinetic study of Caucasian rosuvastatin showed:
Absorption: The blood concentration peaked after 5 hours of oral administration. The absolute bioavailability is 20%.
Distribution: Rosuvastatin is ingested in large amounts by the liver, which is the main site of cholesterol synthesis and LDL-C clearance. The distribution volume of rosuvastatin is about 134L. The plasma protein binding rate (mainly albumin) of rosuvastatin is about 90%.
Metabolism: Limited metabolism of rosuvastatin (about 10%). In vitro metabolic studies with human hepatocytes have shown that rosuvastatin is a weak substrate for cytochrome P450 metabolism. The major isozymes involved in metabolism are CYP, 2C9, 2C19, 3A4, and 2D6, which are less involved in metabolism. Known metabolites are N-demethyl and lactone metabolites. The activity of the N-demethyl metabolite is 50% lower than that of rosuvastatin, while the lactone metabolite is considered to be clinically inactive.
More than 90% of the inhibitory activity against circulating HMG-CoA reductase is from rosuvastatin.
Excretion: Approximately 90% of the dose of rosuvastatin is excreted in the original form with feces (including absorbed and unabsorbed actives) and the remainder is excreted in the urine. About 5% of the urine is the original shape. The plasma elimination half-life is approximately 19 hours. The elimination half-life does not increase with increasing dose. The geometric mean of plasma clearance was approximately 50 L/hr (variation coefficient 21.7%). Like other HMG-CoA reductase inhibitors, uptake of rosuvastatin by the liver involves the membrane transporter OATP-C. This transporter is important in the liver clearance of rosuvastatin.
Linearity: Systemic exposure to rosuvastatin increased proportionally with dose. The pharmacokinetic parameters after multiple administrations did not change.
Only about 10% of the oral dose of rosuvastatin is metabolized, mainly the N-demethylation.
Special population:
Age and gender: Age or gender does not have a clinically significant effect on the pharmacokinetics of rosuvastatin.
Renal insufficiency: In a study abroad for patients with varying degrees of renal impairment, mild and moderate renal disease had no effect on plasma concentrations of rosuvastatin or N-desmethyl metabolites. However, compared with healthy volunteers, patients with severe renal impairment (creatinine clearance <30 ml/min) had a 3-fold increase in plasma concentration and a 9-fold increase in plasma concentrations of N-desmethyl metabolites. The steady-state plasma concentration of rosuvastatin in hemodialysis patients is about 50% higher than that of healthy volunteers.
Hepatic insufficiency: In a study of patients with varying degrees of hepatic impairment in foreign countries, there was no evidence that subjects with a Child-Pugh score of no more than 7 had increased exposure, but 2 cases had a Child-Pugh score of 8 In patients with -9, their exposure to rosuvastatin was at least 2-fold higher than those with lower Child-Pugh scores. There is no experience with subjects with a Child-Pugh score of over 9.
Ethnicity: Foreign pharmacokinetic studies have shown that the median and peak concentration (Cmax) of the area under the plasma concentration-time curve (AUC) of Asian (including Chinese) subjects is about that of Western Caucasians. 2 times. Population pharmacokinetic analysis showed. There were no clinically relevant differences in pharmacokinetics between the Caucasian and black groups.
Studies have shown that after a single dose of Chinese healthy volunteers, the median tmax ranged from 2.5 to 5 hours, followed by an exponential decrease. The half-life (t1/2) is about 11 to 12 hours. On the third day of multiple administration, the plasma concentration reached steady state. The drug accumulation after multiple administrations is small. And regardless of the dose.
【Storage】shading, sealed, and stored in a cool place (not more than 20 ° C).
【Packing】Double aluminum packaging, 7 pieces / box, 14 pieces / box, 21 pieces / box, 28 pieces / box.
【Validity Period】24 months
【Executive Standards】 National Food and Drug Administration Standard YBH04172008
【Approval Number】
(1) 5mg: Chinese medicine quasi-word H20080240;
(2) 10mg: National Drug Standard H20080241;
(3) 20mg: National Drug Standard H20080242.
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 8336337 (Quality Management Department)
Fax number: 0539-8336029 (Sales) 8336338 (Quality Management Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
