
Azithromycin Dispersible Tablets
DescriptionFull Analysis capability
Thin Film Coating Lab
Azithromycin Dispersible Tablets
Upper tract infections such as otitis media, sinusitis, pharyngitis, tonsillitis; lower respiratory tract infections such as bronchitis and pneumonia. Skin and soft tissue infections; simple genital infections caused by Chlamydia trachomatis; simple genital infections caused by non-multidrug-resistant gonococcal bacteria (recombinant infection of melphalan spp).
Clinical pharmacology
Azithromycin is an azalide antibiotic whose mechanism of action is to interfere with the synthesis of its protein (without affecting the synthesis of nucleic acids) by binding to the subunit of the 50s ribosome of sensitive microorganisms. Both in vitro and clinical studies have shown that azithromycin has antibacterial effects against a variety of pathogenic bacteria: Gram-positive aerobic microorganisms: Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Streptococcus hemolyticus. Azithromycin is cross-resistant to erythromycin-resistant Gram-positive bacteria. Most Streptococcus faecalis (Enterococcus) and methicillin-resistant Staphylococcus are resistant to this product. Gram-negative aerobic microorganisms: Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma trachomatis. In vitro and clinical studies have confirmed that azithromycin can prevent diseases caused by the intracellular Mycobacterium complex (composed of Mycobacterium avium and Mycobacterium intracellulare). Azithromycin is ineffective against strains producing beta-lactamase. Studies have shown that azithromycin has an in vitro antibacterial effect on the following microorganisms, but its clinical significance is still unclear, including Streptococcus (C, F, G), Streptococcus mutans, Bordetella pertussis, Haemophilus ducrei, Legionella pneumophila , Bacteroides, Streptococcus pneumoniae, Borrelia, Chlamydia pneumoniae, Treponema pallidum, Ureaplasma urealyticum and the like.
Dosage
This product can be swallowed directly with water, or put into an appropriate amount of warm water and stir well before taking it. (See instructions for details)
Formulation
Dispersible film
specification
0.25g
Instruction manual
Approved date May 8, 2007
Revision date October 1, 2010
December 11, 2013 2015-11-25 increase packaging specifications
Azithromycin dispersible tablet
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Generic Name: Azithromycin Dispersible Tablets
Product Name: Jun Jie
English name: Azithromycin Dispersible Tablets
Chinese Pinyin: Aqimeisu Fensanpian
【Ingredients】The main ingredient of this product is azithromycin.
Chemical name: (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl- α-L-nuclear-hexyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[ [3,4,6,-Trideoxy-3-(dimethylamino)-β-D-wood-pyranosyl]oxy]-1-oxa-6-azacyclopentadecane-15one .
Chemical Structure:
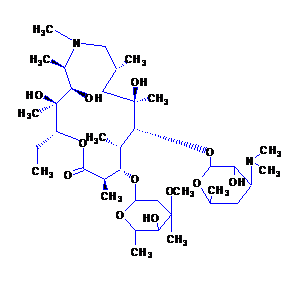
Molecular formula: C38H72N2O12
Molecular weight: 749.00
【Properties】 This product is white or off-white.
[Indications] This product is suitable for the following infections caused by sensitive bacteria:
Upper tract infections such as otitis media, sinusitis, pharyngitis, tonsillitis; lower respiratory tract infections such as bronchitis and pneumonia. Skin and soft tissue infections; simple genital infections caused by Chlamydia trachomatis; simple genital infections caused by non-multidrug-resistant gonococcal bacteria (recombinant infection of melphalan spp.).
【Specification】 0.25g
【Usage and Dosage】Azithromycin tablets for the treatment of infectious diseases, the course of treatment and use are as follows:
This product can be swallowed directly with water, or put into an appropriate amount of warm water and mix well before taking.
Adult: Sexually transmitted diseases caused by Chlamydia trachomatis or sensitive gonococcal bacteria, only need to take 1.0g (4 tablets) of this product in a single dose.
Treatment of other infections: The total dose is 1.5g (6 tablets), divided into three doses, 0.5g (2 tablets) of this product once a day. Or the total dose is the same, still 1.5g (6 tablets), take 0.5g (2 tablets) on the first day, and then take 0.25g (1 tablet) of this product once a day for the second to fifth day.
【Adverse reactions】
(1) Clinical trial experience
Since clinical trials are performed under different conditions, the adverse reaction rate of one drug observed in clinical trials cannot be directly compared with the adverse reaction rates of other drugs in clinical trials, and may not reflect adverse reactions in practical applications. rate.
In the clinical trial of azithromycin intravenous preparation for community-acquired pneumonia, 2 to 5 doses were administered intravenously, and most of the reported adverse reactions were mild to moderate, and recovered after stopping the drug. Most patients in these clinical trials have more than one comorbidity and need to apply other drugs. About 1.2% of patients who received intravenous injections of this product discontinued medication, and 2.4% of patients treated with intravenous or oral azithromycin discontinued medication due to adverse reaction symptoms or abnormal laboratory tests.
In clinical trials in patients with pelvic inflammatory disease, 2% of patients who received azithromycin monotherapy received intravenous drug administration, 2% of patients discontinued due to clinical adverse reactions, and patients with azithromycin and metronidazole 4% of patients discontinued treatment due to adverse reactions.
In the above studies, the most common adverse reactions leading to discontinuation of the drug were gastrointestinal reactions (abdominal pain, nausea, vomiting, diarrhea, etc.) and rashes, leading to abnormal laboratory tests for discontinuation of aminotransferase and/or alkaline phosphatase. high.
In the community-acquired pneumonia study, the most common adverse reaction in adult patients receiving intravenous/oral preparations was gastrointestinal reactions, including diarrhea or loose stools (4.3%), nausea (3.9%), and abdominal pain (2.7). %), vomiting (1.4%). About 12% of patients had adverse reactions related to intravenous injection, the most common being injection site pain (6.5%) and local inflammatory response (3.1%).
In clinical trials of patients with pelvic inflammatory disease, adult female patients receive intravenous/oral preparations of this product. The most common adverse reactions associated with treatment are gastrointestinal reactions, among which diarrhea (8.5%) and nausea (6.6) are common. %), followed by vaginitis (2.8%), abdominal pain (1.9%), anorexia (1.9%), rash and itching (1.9%). In these studies, a combination of azithromycin and metronidazole occurred in a higher proportion of female patients with nausea (10.3%), abdominal pain (3.7%), vomiting (2.8%), site of administration, stomatitis, dizziness, and difficulty breathing. 1.9%).
Other adverse reactions caused by azithromycin intravenous/oral multi-dose regimen did not exceed 1%.
Adverse reactions with an incidence of no more than 1% are:
Gastrointestinal reactions: indigestion, bloating, mucositis, oral candidiasis and gastritis.
Nervous system: headache, lethargy.
Allergic reaction: bronchospasm.
Special feeling: the taste is wrong.
(II) Experience after application after listing
Oral azithromycin preparations are used in adult and/or child patients after marketing, and there are reports of the following adverse events, but it is not certain whether it is caused by azithromycin:
Allergies: joint pain, edema, urticaria, angioedema.
Cardiovascular: Arrhythmias include ventricular tachycardia, hypotension, rare QT interval prolongation, and torsades de point ventricular tachycardia.
Gastrointestinal tract: anorexia, constipation, indigestion, bloating, vomiting/diarrhea but rarely causes dehydration, pseudomembranous colitis, pancreatitis, oral candidiasis, pyloric stenosis and rare tongue discoloration.
Systemic reactions: fatigue, paresthesia, fatigue, discomfort, and anaphylactic shock response.
Genitourinary system: interstitial nephritis, acute renal failure, vaginitis.
Hematopoietic system: thrombocytopenia.
Liver/biliary: The adverse effects associated with liver dysfunction have been reported in the experience of azithromycin after marketing.
Nervous system: convulsions, dizziness/vertigo, headache, lethargy, hyperactivity, nervousness, agitation, and syncope.
Abnormal ears and lost: deafness, tinnitus, hearing loss, dizziness.
Spirit: Aggressive reactions and anxiety.
Skin and Accessories: Itching, a rare and severe skin reaction including erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrosis.
Special Sense: Hearing impairment includes hearing loss, deafness and/or tinnitus, as well as reports of taste/olfactory abnormalities and/or loss.
Laboratory check abnormalities:
Significant laboratory tests (whether or not related to drugs) seen in clinical trials are:
The incidence rate is 4% to 6%: alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine.
The incidence rate is 1% to 3%: lactate dehydrogenase (LDH) and bilirubin are elevated.
The incidence is less than 1%: leukopenia, neutropenia, decreased platelet count, and elevated serum alkaline phosphatase.
Follow-up found that the above laboratory abnormalities were reversible.
In a clinical trial of multiple doses of azithromycin (intravenous/oral) in more than 750 patients, no more than 2% of patients discontinued azithromycin due to treatment-related liver enzyme abnormalities.
【taboo】
It is known to be banned in patients who are allergic to azithromycin, erythromycin, other macrolides or ketolides. Patients with a history of cholestatic jaundice/liver dysfunction after azithromycin use were previously banned.
caveat
Allergic reaction
Reports of severe allergic reactions, including angioedema, anaphylactic shock, and skin reactions, including Stevens Johnson syndrome and toxic epidermal necrolysis, are rare in the treatment with azithromycin. Although rare, there are reports of death. In some patients, allergic symptoms are initially given symptomatic treatment. If the treatment is stopped prematurely, even if azithromycin is not used, the allergic symptoms can recur quickly. It is necessary to extend the time for symptomatic treatment and observation for such patients. It is not known whether the occurrence of these events is related to the long half-life of azithromycin in the tissue and the longer exposure of the body to the antigen.
In the event of an allergic reaction, the drug should be discontinued immediately and given appropriate treatment. The doctor should know that allergic symptoms may reappear after stopping symptomatic treatment.
Hepatotoxicity
There have been reports of abnormal liver function, hepatitis, cholestasis of jaundice, liver necrosis, and liver failure, some of which may be fatal. If symptoms and signs of hepatitis appear, stop using this product immediately.
Clostridium difficile-associated diarrhea
Almost all antibacterial drugs have reports of Clostridium difficile-associated diarrhea (CDAD), including this product, which can range from mild diarrhea to fatal colitis. Antimicrobial treatment can cause changes in the normal flora in the colon, leading to excessive reproduction of Clostridium difficile.
Toxin A and toxin B produced by Clostridium difficile are associated with the pathogenesis of CDAD. Highly toxic C. difficile causes increased morbidity and mortality, and these infections may be difficult to treat with antibiotics and may require colectomy. For all patients with diarrhea after using antibiotics, the possibility of CDAD must be considered. Since there have been reports of CDAD after more than 2 months of antibiotic treatment, it is necessary to carefully ask about the medical history.
If the CDAD is suspected or diagnosed, it may be necessary to discontinue the antibiotic that is not being used for Clostridium difficile. Water, electrolytes, and proteins must be properly supplemented according to clinical needs, and antibiotics that are effective against Clostridium difficile should be given, and surgical evaluation should be performed if necessary.
【Precautions】
General: Because azithromycin is mainly cleared by the liver, patients with impaired liver function should use azithromycin with caution. Subjects with a GFR <10 mL/min have limited data, and azithromycin should be used with caution in such patients. There have been reports of abnormal liver function, hepatitis, cholestatic jaundice, liver necrosis and liver failure, some of which may be fatal. If there are signs and symptoms of hepatitis, azithromycin should be discontinued immediately.
QT interval extension
It has been reported that the use of other macrolide antibiotics, including azithromycin, can cause ventricular repolarization and prolongation of the QT interval, leading to the risk of arrhythmia and torsade ventricular tachycardia. In the post-marketing surveillance of patients taking azithromycin, there was a spontaneous report of a case of torsades de pointes ventricular tachycardia. When weighing the risks and benefits of using azithromycin in high-risk populations, health care providers should consider the risk of potentially fatal QT interval prolongation, including:
· Patients with prolonged QT interval, torsade de pointive ventricular tachycardia, congenital QT prolongation syndrome, bradyarrhythmia or decompensated heart failure are known.
· Patients who are known to prolong their QT interval medications, such as antipsychotics, antidepressants, and fluoroquinolones.
· Patients with arrhythmia, such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and receiving type IA (quinidine, procainamide) and type III (Dofetilide, amiodarone, sotalol) patients with antiarrhythmic drugs.
· Elderly patients: Elderly patients may be more sensitive to drug-related QT interval effects.
Azithromycin-treated patients have reported aggravation of myasthenia gravis or a new myasthenia gravis syndrome.
In the absence of a diagnosis or a high degree of suspected bacterial infection, or no indication of prevention, the use of this product may not be beneficial to the patient, and will increase the risk of drug-resistant bacteria.
Patients need to know:
Azithromycin should be discontinued immediately when any signs of allergic reactions occur and contact your doctor.
Patients should be advised that antibiotics including this product (azithromycin) can only be used to treat bacterial infections and cannot be used to treat viral infections (eg the common cold). When using this product (azimycin) to treat bacterial infections, patients must be informed that although they usually feel better at the beginning of treatment, they should follow the doctor's instructions for accurate medication. Missing or not completing the entire course of treatment may:
(1) reduce the efficacy of current treatments;
(2) increase the possibility of bacterial resistance, which will lead to the inability of azithromycin or other antibiotics to treat these resistant bacteria in the future.
Antibiotic treatment often causes diarrhea, which usually recovers after stopping the antibiotic. Sometimes, after antibiotic treatment, the patient may have a watery stool or bloody stool (with or without stomach cramps and fever) 2 months or more after the last antibiotic use. If this happens, the patient should contact the doctor as soon as possible.
【Pregnant women and lactating women】
Animal reproductive toxicity studies have shown that azithromycin crosses the placenta but shows no signs of damage to the litter. There is no secretion data of this product in breast milk. Animal test data does not fully predict the application of humans. The safety of use in pregnancy and lactation has not been confirmed so far, so this product should not be used when there is no suitable choice for pregnant or lactating women.
【Child medication】This experiment was not performed and there is no reliable reference.
【Geriatric Use】This experiment was not performed and there is no reliable reference.
【medicine interactions】
Drug interactions: Oral administration of a single dose of azithromycin in the steady state of nelfinavir can increase the serum concentration of azithromycin. Although there is no need to adjust the dose of azithromycin when used in combination with nelfinavir, the known side effects of azithromycin such as liver enzyme abnormalities and hearing impairment must be closely monitored.
Spontaneous post-marketing reports suggest that the combined use of azithromycin may enhance the effects of oral anticoagulants. When patients are combined with azithromycin and oral anticoagulant drugs, prothrombin time should be closely monitored.
At a therapeutic dose, azithromycin versus atorvastatin, carbamazepine, cetirizine, didanosine, efavirenz, fluconazole, indinavir, midazolam, rifabutin, west The pharmacokinetics of dipyridamole, theophylline (intravenous and oral administration), triazolam, trimethoprim/sulfamethoxazole or zidovudine have little effect. When used together, efavirenz or fluconazole has little effect on the pharmacokinetics of azithromycin. When azithromycin is combined with any of the above drugs, it is not necessary to adjust the dose of either drug.
Azithromycin has not been reported to interact with the following drugs in clinical trials. However, no specific studies have been conducted to date to evaluate the potential interaction between azithromycin and these drugs. However, these conditions have occurred when other macrolides were used. Therefore, in the absence of new research data, azithromycin should be closely observed when combined with the following drugs:
The blood concentration of digoxin-digoxigenin increased.
Ergosamine or dihydroergotamine - acute ergot poisoning, manifested as severe peripheral vasospasm and dysesthesia.
Terbinadine, cyclosporine, hestobeta and phenytoin were elevated in concentration.
Impact on laboratory tests: No reports of effects on laboratory tests have been reported.
【Drug overdose】 The adverse effects of drug overdose are the same as the recommended dose. Once overuse occurs, symptomatic and supportive care can be given depending on the condition, such as gastric lavage or general supportive therapy.
【Pharmacology and Toxicology】
Pharmacological action
Azithromycin is an azalide antibiotic whose mechanism of action is to interfere with the synthesis of its protein (without affecting the synthesis of nucleic acids) by binding to the subunit of the 50s ribosome of sensitive microorganisms.
Both in vitro and clinical studies have shown that azithromycin has an antibacterial effect on a variety of pathogenic bacteria:
Gram-positive aerobic microorganisms: Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Streptococcus hemolyticus.
Azithromycin is cross-resistant to erythromycin-resistant Gram-positive bacteria. Most Streptococcus faecalis (Enterococcus) and methicillin-resistant Staphylococcus are resistant to this product.
Gram-negative aerobic microorganisms: Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma trachomatis.
In vitro and clinical studies have confirmed that azithromycin can prevent diseases caused by the intracellular Mycobacterium complex (composed of Mycobacterium avium and Mycobacterium intracellulare).
Azithromycin is ineffective against strains producing beta-lactamase.
Studies have shown that azithromycin has an in vitro antibacterial effect on the following microorganisms, but its clinical significance is still unclear, including Streptococcus (C, F, G), Streptococcus mutans, Bordetella pertussis, Haemophilus ducrei, Legionella pneumophila , Bacteroides, Streptococcus pneumoniae, Borrelia, Chlamydia pneumoniae, Treponema pallidum, Ureaplasma urealyticum and the like.
Toxicological research
Genotoxicity Results of human lymphocyte assay, mouse bone marrow micronucleus assay and mouse in vitro lymphoma cell assay Azithromycin showed no mutagenic effects.
Reproductive toxicity Reproductive toxicity tests in rats and mice have shown that when azithromycin (oral administration) doses reach a dose level that produces moderate maternal toxicity (ie 200 mg/kg/day, about 500 mg of human dose based on body surface area) No teratogenic effect was observed when /kg/day was 2 to 4 times.
No damage to fertility and fetus has been found. There are currently no adequate and rigorous controlled clinical trials in pregnant women. Since the results of animal reproduction studies do not always predict human conditions, azithromycin can only be used by pregnant women when it is really necessary. It is not known whether this product is secreted in human milk. Since many drugs are secreted by human milk, women who are breast-feeding should pay attention to it when using it.
Carcinogenicity There are no studies on the carcinogenicity of long-term use of azithromycin animals.
【Pharmacokinetics】
It is rapidly absorbed after oral administration and has a bioavailability of 37%. After a single dose of 0.5 g orally, the peak time is 2.5 to 2.6 hours, and the peak plasma concentration (Cmax) is 0.4 to 0.45 mg/L. This product is widely distributed in the body, the concentration in each tissue can reach 50 times of the blood concentration in the same period, and the concentration in macrophages and fibroblasts is high. The former can transport azithromycin to the inflammation site. The blood elimination half-life (T1/2) after single-agent administration is 35 to 48 hours, and more than 50% of the administered amount is discharged through the biliary tract in the original form, and about 4.5% in the original form is discharged through the urine within 72 hours after administration. The serum protein binding rate of this product is reduced with the increase of blood concentration. When the blood concentration is 0.02μg/ml, the serum protein binding rate is 15%. When the blood concentration is 2μg/ml, the serum protein binding rate is 7%. Food does not affect the bioavailability of this product.
【Storage】shading, sealing, and storing in a dry place.
【Packing】 aluminum plastic packaging, 6 pieces / box, 12 pieces / box.
【Validity period】24 months.
【Executive Standards】The second edition of the 2010 edition of the Chinese Pharmacopoeia.
【Approval No.】National Drug Standard H20020729.
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 0539-8336337 (Quality Pipe Department)
Fax number: 0539-8336029 (Sales) 0539-8336338 (Quality Pipe Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
