Mosapride Citrate Tablets
DescriptionFull Analysis capability
Thin Film Coating Lab
Mosapride Citrate Tablets
Indication
This product is a digestive tract motility agent, mainly used for functional dyspepsia with heartburn, belching, nausea, vomiting, early satiety, upper abdominal augmentation and other gastrointestinal symptoms; can also be used for gastroesophageal reflux disease, diabetes Gastric dysfunction in patients with gastroparesis and partial gastrectomy.
Clinical pharmacology
This product is a selective serotonin 4 (5-HT4) receptor agonist, which promotes the release of acetylcholine by exciting the gastrointestinal cholineergic interneurons and the 5-HT4 receptor of the myenteric plexus, thereby enhancing the stomach. The movement of the intestine improves the gastrointestinal symptoms of patients with functional dyspepsia and does not affect the secretion of gastric acid. This product has no affinity with the dopamine D2, 5-HT1, and 5-HT2 receptors on the synaptic membrane of the brain, and thus has no extrapyramidal side effects caused by these receptor blockages.
Dosage
Oral, 1 tablet at a time, 3 times a day, taken before meals.
Formulation
tablet
specification
5mg.
Instruction manual
Approval date:
Modified date:
Mosapride citrate tablets
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Common name: mosapride citrate tablets
Product Name: Quick Force
English name: Mosapride Citrate Tablets
Pinyin: Juyuansuan Moshabili Pian
【Ingredients】 Mosapride citrate.
Chemical name: 4-amino-5-chloro-2-ethoxy-N-{[4-(4-fluorobenzyl)-2-morpholinyl]methyl}benzamide decanoate.
Chemical Structure:
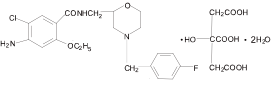
Molecular formula: C21H25ClFN3O3·C6H8O7·2H2O Molecular weight: 650.05
【Properties】 This product is white or off-white.
【indications】
This product is a digestive tract motility agent, mainly used for functional dyspepsia with heartburn, belching, nausea, vomiting, early satiety, upper abdominal augmentation and other gastrointestinal symptoms; can also be used for gastroesophageal reflux disease, diabetes Gastric dysfunction in patients with gastroparesis and partial gastrectomy.
【Specification】5mg.
【Usage and Dosage】 Oral, 1 tablet at a time, 3 times a day, taken before meals.
【Adverse reactions】
Mainly manifested as diarrhea, abdominal pain, dry mouth rash and burnout, dizziness and so on. Occasionally, eosinophilia, elevated triglycerides, and aspartate aminotransferase (GOT), alanine aminotransferase (GPT), alkaline phosphatase (ALP), and γ-glutamyltranspeptidase (GGT) are elevated.
【Contraindications】 Disabled for allergic to this product.
【Precautions】 When taking a period of time (usually 2 weeks), if the symptoms of the digestive tract have not changed, stop taking it.
【Pregnant women and lactating women】This experiment was not conducted and there is no reliable reference.
【Child medication】 This experiment was not performed and there is no reliable reference.
【Geriatric Use】 Elderly patients should pay attention to observation and find that side effects should be properly treated immediately, such as reducing the dose.
【Drug interactions】 Combined with anticholinergic drugs (such as atropine sulfate, scopolamine bromide, etc.) may attenuate the effects of this product.
【Drug overdose】This experiment was not performed and there is no reliable reference.
【Pharmacology and Toxicology】
This product is a selective serotonin 4 (5-HT4) receptor agonist, which promotes the release of acetylcholine by exciting the gastrointestinal cholineergic interneurons and the 5-HT4 receptor of the myenteric plexus, thereby enhancing the stomach. The movement of the intestine improves the gastrointestinal symptoms of patients with functional dyspepsia and does not affect the secretion of gastric acid. This product has no affinity with the dopamine D2, 5-HT1, and 5-HT2 receptors on the synaptic membrane of the brain, and thus has no extrapyramidal side effects caused by these receptor blockages.
In the toxicological test, the LD50 of oral mosapride in mice was 2004 mg/kg, and the LD50 of intraperitoneal injection was 587.77 mg/kg.
【Pharmacokinetics】
This product is mainly absorbed from the gastrointestinal tract. The distribution of the drug in the stomach, liver and kidney is the highest, followed by plasma, and there is almost no distribution in the brain. A healthy adult took 5 mg of this product on an empty stomach and absorbed rapidly. The peak concentration of blood drug was 30.7 ng/ml, the peak time was 0.8 hours, the half-life was 2 hours, and the plasma protein binding rate was 99.0%. This product is metabolized by CYP3A4 enzyme in cytochrome P-450 in the liver. Its main metabolite is de-4-fluorobenzyl mosapride. This product is mainly excreted in urine and feces.
【Storage】 sealed, kept in a cool place (not more than 20 ° C) in a dry place.
【Packing】aluminum plastic packaging. 24 pieces / box, 36 pieces / box, 48 pieces / box.
【Validity period】24 months.
【Executive Standards】 National Food and Drug Administration National Drug Standard WS1-(X-287)-2003Z.
【Approval No.】National Medicine Zhunzi H19990317.
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 0539-8336337 (Quality Pipe Department)
Fax number: 0539-8336029 (Sales) 0539-8336338 (Quality Pipe Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
